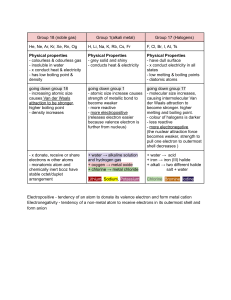
IGCSE Resources Revision and Formula Sheet For Cambridge IGCSE Chemistry (0620) - by _peacegod. Chemical Reactions ● Contact Process: ○ 2SO₂(g) + O₂(g) ⇌ 2SO₃(g) ○ Source of SO₂: ■ Burning sulfur in air ● S(s) + O₂(g) → SO₂(g) ■ Roasting sulfide ores such as Zinc blende (ZnS). ● 2ZnS(s) + 3O₂(g) → 2ZnO(s) + 2SO₂(g) ○ Conditions required: ■ 450 °C ■ 200k Pa / 2 atm ■ Vanadium (V) Oxide catalyst ● Haber Process ○ N₂(g) + 3H₂(g) ⇌ 2NH₃(g) ○ Conditions required: ■ 450 °C ■ 20 000kPa / 200 atm ■ Iron catalyst ● Metals ○ ○ ○ Acid + metal carbonate → salt + H₂O + CO₂ Acid + alkali → salt + H₂O(l) Metal + acid → salt + H₂(g) Metal + water → metal hydroxide + hydrogen ■ Only metals such as Potassium, Sodium and calcium react with water. (Mg does too but slowly) ■ Rest of the metals react with steam to produce Metal oxide + hydrogen gas Acid + metal oxide → salt + H₂O(l) Metal oxide/hydroxide + ammonium salt → salt + ammonia(g) + H₂O(l) ZnO(s) + 2HCl(aq) → ZnCl₂(aq) + H₂O(l) ○ ○ ZnO(s) + 2NaOH(aq) → Na₂ZnO₂(aq) + H₂O(l) Zinc oxide + sodium hydroxide → sodium zincate + water ○ ○ Al₂O₃(s) + 2NaOH(aq) → 2NaAlO₂(aq) + H₂O(l) Aluminium oxide + sodium hydroxide → sodium illuminate + water ○ ○ ○ ○ ● Hydration (Alkene to Alkanol) ○ ○ Catalytic addition reaction of steam Conditions required: ■ 300 °C ■ 6000kPa / 60 atm ■ (Acidified) Phosphoric (V) acid catalyst Made By _peacegod. 1 ● Complete and Incomplete combustion ○ ○ When burned in a good supply of air the products are CO₂ and H₂O(g). When burned in a limited supply of air the products are CO and H₂O(g). ● Hydrogenation ○ Conditions required: ■ 200°C. ■ Nickel catalyst. ● Bromination (Test for unsaturation) ○ Aqueous bromine turns colorless when reacted with an alkene. ○ Unsaturated compounds have molecules in which one or more carbon–carbon bonds are not single bonds. ● Cracking ○ Conditions required: ■ 500°C ■ Alumina or Silica catalyst ○ Decane → octane + ethene ○ C₁₀H₂₂ → C₈H₁₈ + C₂H₄ ○ Note: ■ Two or more alkenes and hydrogen will be formed. ■ Or an alkane and an alkene (both short chains). ● Oxidation (Ethanol to Ethanoic acid) ○ Conditions required: ■ (Acidified) Aqueous Potassium Manganate (VII) ■ KMnO₄ (aq) ● Substitution reaction with Chlorine ○ Photochemical reaction ■ The activation energy required to start the reaction is provided by UV light (sun light). ■ Methane + chlorine → chloromethane + hydrogen chloride ■ CH₄(g) + Cl₂(g) → CH₃Cl(g) + HCl(g) ● Oxides of Nitrogen ○ N₂(g) + O₂ → 2NO(g) ○ 3NO₂(g) + H₂O(l) → 2HNO₃(aq) + NO(g) ● Photosynthesis ○ Carbon dioxide + water → glucose + oxygen ○ 6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂ Made By _peacegod. 2 ● Fermentation ○ Anaerobic respiration. ■ Takes place in the presence of yeast and in the absence of oxygen. ○ Catalysed by enzymes in the yeast. ○ Temperature required: between 25 and 35 °C. ○ Aqueous glucose (enzymes)→ ethanol + carbon dioxide ○ C₆H₁₂O₆ (aq) → 2C₂H₅OH (aq) + 2CO₂ (g) ● Metals Extraction ○ the burning of carbon (coke) to provide heat and produce carbon dioxide. ○ C + O₂ → CO₂ ○ the reduction of carbon dioxide to carbon monoxide. ○ C + CO₂ → 2CO ○ the reduction of iron(III) oxide by carbon monoxide. ○ Fe2O₃ + 3CO → 2Fe + 3CO₂ ○ the thermal decomposition of calcium carbonate /limestone to produce calcium oxide. ○ CaCO₃ → CaO + CO₂ ○ the formation of slag ○ CaO + SiO₂ → CaSiO₃ (Calcium Silicate) ○ Why is coke added ? ■ Coke releases heat. ■ Coke reduces iron(III) oxide. ■ Coke reacts with carbon dioxide to form carbon monoxide. ○ Role of cryolite ■ Acts as a solvent. ■ Improves conductivity. ■ Lowers the operating temperature. ○ Why do carbon anodes need to be replaced regularly ? ■ At high temperature, the oxygen reacts with the carbon in the electrodes forming CO2. The carbon anodes then slowly burn away and have to be replaced frequently. ○ The reactions at the electrodes: ■ Al³⁺(l) + 3e⁻ → Al(l) ■ 2O²⁻(l) → O₂(g) + 4e⁻ Made By _peacegod. 3