Gas Laws: Boyle's, Charles's, Ideal Gas Law Explained
advertisement

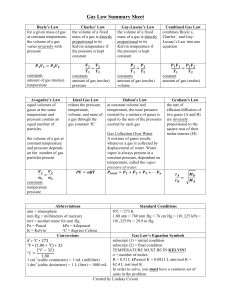
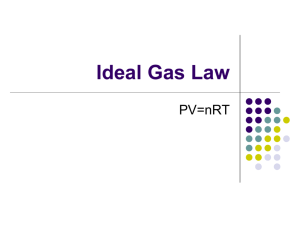
Gas Laws • The four properties of gases (amount in moles, volume, temperature, pressure) are all related. A change in any of these will affect one or more of the others. • The gas laws show the relationship between individual properties and all properties together. • The first three of these gas laws relate only two variables. Gas Laws • 3 of the gas laws deal with the relationship between just two gas properties at a constant number of gas moles. • Boyle’s Law says that at constant T that as the pressure increases on a gas the volume decreases. • Charles‘ Law says that at constant P that as the temperature increases the volume increases. • Gay-Lussac’s Law says that at a constant V that as the temperature increases the pressure increases. Gas Laws • These 3 laws can be brought together into an equality equation called the combined gas law. • The relationship between pressure, volume, and temperature is fixed at constant moles. • PV/T = a constant value • That constant value does not change during individual changes in the properties. • Therefore (PV/T)i = (PV/T)f i and f are initial and final Gas Laws • Attention must be paid to appropriate units in gas equations. • Pressure can be in any unit as long as it in consistently applied. • Volume can be in any unit as long as it is consistently applied • Temperature MUST be in Kelvin because it is the only absolute temperature scale. • Amount of gas is in moles. Gas Laws • One more gas law exists for changes in the gas properties as well as the amount of gas present in moles. • This law is referred to as the Ideal Gas Law because it accurately predicts gas properties of ideal gases. Most gases at moderate conditions behave like an ideal gas. • Ideal Gas Law – PV = nRT – R is the gas constant having a value of .0821 if using liters, atm, mol, and kelvin temperature.