Reaction Practice
advertisement

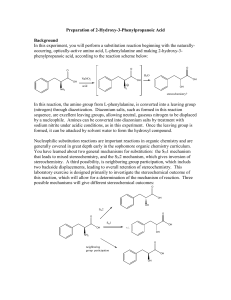
Reaction Practice taken from previous exams, and the online lecture notes. It will help. 1. Show the steps necessary to convert cyclopentanol to a Grignard reagent. OH 2 The following reaction gives three possible products. Give the structure for each. KOH Ethanol Br 3. Give the product reactions. 1) Hg(OAc)2 2) NaBH4 1) BH3 2) H2O2 4. Give the Products for the following reactions. H2, Pd OsO4, H2O NaBH4 H C C H H O3 Zn, HOAc 5. Show all steps needed to make 2,2-dibromo-2-butane from 2-butene. 6. Identify the reagents a and b in the following scheme. 7. Refer to Structures 10-1 8. In the discussion on relative reactivity of alkane hydrogens towards radical chlorination, we showed that the relative rate of 2 to 1 hydrogen atom abstratction is 3.5 : 1 for butane. Calculate the relative amounts of 1-chloropropane and 2-chloropropane obtained the radical chlorination of propane, using this relative rate of reactivity. 9. Using acetylene and any alkyl halides as starting materials, synthesize acetic acid. More than one step may be required. Show all reagents and all intermediate compounds in your synthetic scheme. 10. What reagents could be used to make the following transformation? 11. Predict the product of the reaction below. Be sure to indicate stereochemistry when appropriate. 12. Predict the product of the reaction below. Be sure to indicate stereochemistry when appropriate. 13. Predict the product of the reaction below. Be sure to indicate stereochemistry when appropriate. 14. Predict the product of the reaction below. Be sure to indicate stereochemistry when appropriate. 14. Predict the products of each reaction. Indicate regiochemistry and stereochemistry when relevant. 15. Predict the products of each reaction. Indicate regiochemistry and stereochemistry when relevant. 16. The sequence of (1) alkene hydroxylation followed by (2) diol cleavage is often an excellent alternative to direct alkene cleavage with ozone. Give the formula for reagent A and B. 17. 18. Draw all the monochlorination products of methylcyclopentane (ignore stereoisomers). 19. Show all reagents and intermediates necessary to carry out the following conversion. 20. Predict the major organic product(s) in each reaction. If more than one major organic product is expected draw each one. HBr KOH 21. Show the steps necessary to convert cyclohexanol to a Grignard reagent. OH 22. Write the complete stepwise mechanism for the following reaction. Show all intermediate structures and all electron flow with arrows. 23. Give the all products expected for the following reaction. Ignore R, S differences. NBS h, CCl4 24. Give the products for each step including the equilibrium product. CH3 C C BH3 H2O2 H2O CH3 25. Show the synthesis of 4-fluoro-2-pentyne from propyne and any organic or inorganic reagents. Include the proper stereochemistry for the other organic reagent. F H3C C C C H CH3 26. Show all steps needed to make 2,2-dibromo-2-butane from 2-butene. 27. Why does the rearrangement that results in the formation of product B occur? 28. Know these: 29. Predict the products of each reaction. Indicate regiochemistry and stereochemistry when relevant. 30. Predict the products or reactants of each reaction. Indicate regiochemistry and stereochemistry when relevant. 31. Give the Products for the following reactions of phenyethene with the indicated reagents. H2, Pd OsO4, H2O NaBH4 H C C H H O3 Zn, HOAc 32. Give the product reactions. 1) Hg(OAc)2 2) NaBH4 1) BH3 2) H2O2 33. The following reaction gives three products, two of which are enantiomers. Give the structure for each and the R and S designations for the enantiomers. CH3 H3C H H H + H Br H 34. Draw a qualitative reaction energy diagram for this reaction. Label the positions of all reactants, intermediates and products. 35. Reaction Pathway 35. Using the curved arrow formalism, show the flow of electrons for this reaction. 36. Be able to show the complete mechanism for the following reaction, complete with arrows showing electron flows. 2-bromo-2-methylpropane See next page for help. If you couldn’t do the previous problem, take a look at the following: Add curved arrows to indicate electron flow for each first step of the following mechanism. 37. Use curved arrows to show the electron flow and write the products of this Lewis acid - base reaction. 38. Using the curved arrow formalism, show the flow of electrons for this reaction. 39. Add curved arrows to the following reaction to indicate the flow of electrons in each. I hope this helps.