Chemical Names & Formulas
advertisement

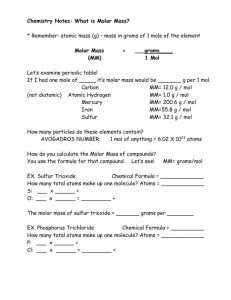
Chemical Names & Formulas 7-1 Chemical formula… • indicates the relative __________ and ____________ of atoms in a compound • Covalent bonding: _____________ formula – H2O 2 atoms H, 1 atom O • Ionic bonding: ______________ ________ (simplest ratio of the cmpd’s pos. & neg. ions) – MgCl2 1 Mg ion, 2 chloride ions How many atoms of each element are in Ba3(PO4)2 ? Monatomic ions • ions formed from ____________________ • page 205 Table 7-1 (know these…don’t need to memorize, however!) Naming monatomic ions 1. Cation: simply the name of the element 2. Anion: drop the ending and add –ide • • NaCl: sodium chloride Li2O: lithium oxide • NaF ______________________ • AlN _______________________ Binary Ionic Compounds • • cmpds composed of 2 different elements NaCl • Rules for naming binary ionic cmpds: 1. Write the symbols side by side..cation first 2. Cross over the charges by using the abs. value of each as the subscript for the other 3. Check…reduce to smallest whole-number ratio, if necessary Write and name the formulas for the following binary ionic cmpds: • lithium and iodine • sulfur and magnesium • zinc and bromine • fluorine and calcium • sodium and oxygen Stock Nomenclature • Roman numerals are used to indicate an ion’s charge when it can form more than one ion • copper (I) chloride Cu1+ • copper (II) chloride Cu+2 Write the formula & name… • copper (I) and oxygen • iron (III) and sulfur • copper (II) and chlorine • tin (II) and chlorine • tin (IV) and sulfur • vanadium (II) and fluorine • vanadium (III) and bromine Polyatomic ions • a “charged” group of atoms that are held together by covalent bonds • i.e. SO42- oxyanions • negative ions that contain oxygen • • • • -ate: most common form of group -ite: one less O than common form hypo: less O than the -ite form per: more O than the -ate form Examples of oxyanions • Given: ClO31- is most common form • ClO21_______________ • ClO1_______________ • ClO41_______________ Write the formula for the following: • copper(II) nitrate • potassium iodide • magnesium hydroxide • ammonium acetate • calcium carbonate • sodium sulfate • iron(III) nitrate Name the following: • Ca(OH)2 • Fe(OH)2 • Na2SO4 • CuSO4 • FeO • Fe2O3 Binary Molecular Compounds • composed of ____ individually bonded (covalent) units • usually cmpds between 2 ______________ • 2 systems: – newer: Stock system – older: Prefix system (see p. 212….know prefixes) Numerical Prefixes • • • • • • • • • • 1 2 3 4 5 6 7 8 9 10 Rules for naming binary molecular cmpds: 1. Write the less electronegative element first. Precede with a prefix only when there are >1 atoms of an element. 2. Write the more electronegative element 2nd, utilizing the –ide as in binary ionic cmpds. Always precede its name with a prefix. 3. The “o” or “a” of the prefix is usually dropped when the element’s name begins with a vowel. • • • • IF3 iodine trifluoride N2O5 dinitrogen pentoxide Naming acids • Ch 15 – learn about acids • The term acid is used when a specific type of cmpd is in a solution of water …. (aq) means aqueous solution • Common oxyacids to know now: • H2SO4 (aq) sulfuric acid • HNO3 (aq) nitric acid • H3PO4 (aq) phosphoric acid • H2CO3 (aq) • __________________________ • CH3COOH (aq) • __________________________ Naming acids • -ate ion forms the –ic acid – H2SO4 sulfuric acid – HNO3 nitric acid – H3PO4 phosphoric acid • -ite ion forms the –ous acid – H2SO3 sulfurous acid – HNO23 nitrous acid – H3PO3 phosphorous acid Binary acids… • HCl hydrochloric acid • HF ________________________ • HBr ________________________ • HI ________________________ Sometimes all of the Hs don’t come off of an acid when it reacts…….. • H2CO3 carbonic acid • HCO31- hydrogen carbonate Diatomic elements: • • • • • • • H2 N2 O2 F2 Cl2 Br2 I2 Oxidation number • also known as __________________ _________ • number assigned to an atom in a molecular cmpd that indicates that general distribution of electrons among the bonded atoms • gives an insight into the combining ability of an atom – not “real” charges but are artificial “bookkeeping” devices to keep track of overall edistribution • Ionic cmpds: the charge on the ion IS the electron distribution ∴ ox. # = charge on ion…physically meaningful info Summary of Ox # Rules (p.216) 1. Pure elements have an ox. # of ____________ i.e. copper, Cu = ox # = ______ oxygen, O2 = ox # = ______ 2. More electroneg. element has an ox. # = charge it would have if it were an __________. Less electroneg. element has an ox # = charge it would have if it were a ____________. i.e. oxygen in H2O O = ox # of ___________ 3. F: _______ oxidation # 4. O: ________ oxidation # except in peroxide (H2O2) where it is –1, and with halogens (OF2) where it is +2. 5. H: ________ except when it’s more ________________. than other atoms (metals….NaH) where it is ________. 6. Algebraic sum of all ox. #s in cmpd = ____. 7. Algebraic sum of all ox. #s in polyatomic ion = ___________________. 8. IF ionic, ox. # ______ charge of the ion Assign oxidation numbers to each element in the following cmpds or ions: • CCl4 • NO2 • LiH • sulfate ion • nitrate ion • H3PO4 • P4O10 Using the newer Stock system, write formulas for the following molecular cmpds: • nitrogen (II) oxide • phosphorus (III) chloride • sulfur (IV) oxide • bromine Formula Masses • sum of the average atomic masses of all the atoms represented in a formula • (can be of a molecule, formula unit or ion) • • • • • Example: Formula mass of H2O: 2 (ave. atomic mass of H) + 1(ave. at. mass of O) = 2(1.01 amu) + 1(16.00 amu) = 2.02 amu + 16.00 amu = 18.02 amu Calculate the formula mass of the following: • sulfuric acid • calcium nitrate • phosphate ion • magnesium chloride Molar Masses • sum of the masses of the elements present in a mole of the molecules or formula units that make up a compound • Molar mass of water, H2O: • 1 mole H2O = 2 mol H + 1 mol O • 2 mol H x 1.01 g H = 2.02 g H 1 mol H • 1 mol O x 16.00 g = 16.00 g O 1 mol O + _____________ • 18.02 g • ∴ Molar mass of water = 18.02 g/mol What is the molar mass of… • aluminum sulfide • sodium nitrate • barium hydroxide Using Molar Mass as a conversion factor between “moles” ↔ “mass” • What is the mass, in grams, of 0.500000 moles of barium hydroxide? • 0.500000 mol Ba(OH)2 x • = 85.675 g Ba(OH)2 = 171.35 g Ba(OH)2 1 mole Ba(OH)2 How many moles are in 6.60 g of ammonium sulfate? Gram-Mole Conversions Remember…. 1 mole = 6.02 x 1023 “things” • How many molecules are in 50.0 g of water? • 50.0 g H2O x 1 mol H2O x ________g H2O __ molecules 1 mol H2O • = __________________ molecules H2O Percentage Composition • % = part x 100 whole • percentage by mass of each element in a cmpd Find the % copper in copper(I) sulfide • % Cu = mass of Cu in 1 mole of Cu2S x 100% mass of 1 mol of Cu2S or… % Cu = mass of Cu in 1 mole of Cu2S x 100% molar mass of Cu2S • Cu2S = _____________ g/mol, and in1 mol Cu2S, there are _______ mol Cu = ___________ g Cu • % Cu = mass of Cu in 1 mole of Cu2S x 100% mass of 1 mol of Cu2S • = _________ g Cu x 100% = ________% Cu in Cu2S g Cu2S What is the percent carbon in ammonium carbonate? Empirical formula • formula that shows the smallest wholenumber ____________ ratio of the different atoms in a compound • In ionic cmpds, the formula unit usually is the empirical formula What is the empirical formula of the following molecular formulas? • Ethyne, C2H2 • Benzene, C6H6 • Glucose, C6H12O6 • Acetic Acid Calculation of an empirical formula • Empirical formula: smallest whole-number MOLE ratio of the different atoms in a compound • ∴ • 1)determine moles of each element • 2)determine the simples whole # ratio of these moles What would the formula be of a cmpd that was composed of 2 mol hydrogen and 1 mol oxygen? What would the formula be of a cmpd composed of 0.78 mol Al & 2.34 mol Br? What is the empirical formula for a cmpd that contains 0.900 g calcium and 1.60 g chlorine? Determine the empirical formula in a cmpd that is 40.0% C, 6.71% H, and 53.3% O. If the empirical formula is CH2 and the molar mass is 42 g/mol, what is the molecular formula? If the molecular mass for the prev. problem (cmpd that is 40.0% C, 6.71% H, and 53.3% O) is 90 g/mol, determine the molecular formula. Do Section Review p. 233 1. 2. 3. 4. 5. Na2SO3 Fe2S3 K2CrO4 N2O5 N2O4