幻灯片 1
advertisement

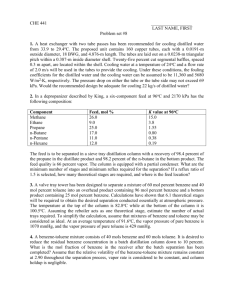
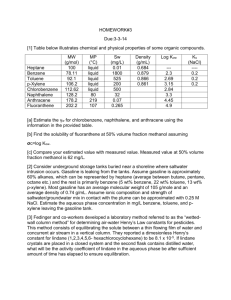
Chapter6 Absorption and Stripping of Dilute Mixtures Exercises Exercise6.1 Prior to 1950, only two types of commercial random packings were in common use: Raschig rings and Berl saddles. Starting in the 1950s, a wide variety of commercial random packings began to appear. What advantages do these newer packings have? By what advances in packing design and fabrication techniques were these advantages achieved? Why were structured packings introduced? Exercise6.2 In Example 6.3, a lean oil of 250 MW is used as the absorbent. Consideration is being given to the selection of a new absorbent. Available streams are: Rate, Density, gpm 1b/gal MW C5s 115 5.24 72 Light oil 36 6.0 130 Medium oil 215 6.2 180 Which stream would you choose? Why? Which streams, if any, are unacceptable? Exercise6.3 A straw oil used to absorb benzene from coke-oven gas is to be steam-stripped in a sieve-plate column at atmospheric pressure to recover the dissolved benzene. Equilibrium conditions at the operating temperature are approximated by Henry's law such that, when the oil phase contains 10 mol% CfiHft, the CeHfi partial pressure above the oil is 5.07 kPa. The oil may be considered nonvolatile. The oil enters containing 8mol% benzene, 75% of which is to be recovered. The steam leaving contains 3 mol% C6H6. (a) How many theoretical stages are required? (b) How many moles of steam are required per 100 mol of oil-benzene mixture? (c) If 85% of the benzene is to be recovered with the same oil and steam rates, how many theoretical stages are required? Exercise6.4 One thousand kilomoles per hour of rich gas at 70°F with 25% C1, 15% C2, 25% C3, 20% nC4, and 15% nC5 by moles is to be absorbed by 500kmol/h of nC10 at 90°F in an absorber operating at 4atm. Calculate by the Kremser method the percent absorption of each component for four, ten, and thirty theoretical stages. What do you conclude from the results? (Note: The K-value of nC10 at 80°F and 4atm is 0.0014.) Exercise6.5 The absorption operation of Examples 6.1 and 6.4 is being scaled up by a factor of 15, such that a column with an 11.5-ft diameter will be needed. In addition, because of the low efficiency of 30% for the original operation, a new tray design has been developed and tested in an Oldershawtype column .The resulting Murphree vapor-point efficiency, EOV, for the new tray design for the system of interest is estimated to be 55%. Estimate EMV and E0. (To estimate the length of the liquid flow path, ZL, use Figure 6.16. Also. assume that u/de = 6 Exercise6.6 Conditions at the bottom tray of a reboiled stripper are as shown in Figure 6.48. If valve trays are used with a 24-in. tray spacing, estimate the required column diameter for operation at 80% of flooding. Exercise6.7 A wastewater stream of 600gpm, containing 10ppm (by weight) of benzene, is to be stripped with air in a packed column operating at 25°C and 2atm to produce water containing 0.005ppm of benzene. The packing is 2-in. Flexirings made of polypropylene. The vapor pressure of benzene at 25°C is 95.2torr. The solubility of benzene in water at 25°C is 0.180 g/100 g. An expert in VOC stripping with air has suggested use of 1,000scfm of air (60°F, 1atm), at which condition one should achieve for the mass transfer of benzene: kLa=0.067s-1 and kga=0.80s-1 Determine: (a) The minimum air stripping rate in scfm. Is it less than the rate suggested by the expert? If not, use 1.4 times your minimum value. (b) The stripping factor based on the air rate suggested by the expert. (c) The number of transfer units, NOG required. (d) The overall mass transfer coefficient, KGa, in units of mol/m3-s-kPa and s-1. Which phase controls mass transfer? (e) The volume of packing in cubic meters.