Aromatic Compounds and Benzene
advertisement

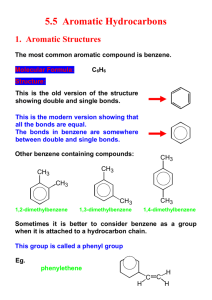
Chapter 12.5 Aromatic Hydrocarbons 1 Aromatic Compounds and Benzene • Aromatic compounds contain benzene. • Benzene, C6H6 , is represented as a six carbon ring with 3 double bonds. • Two possible isomers can be drawn to show benzene in this form. H H H H H H H H H H H H 2 Aromatic Compounds and Benzene • The name aromatic came from many of the first examples discovered were pleasant smelling • Many aromatics that have recently been discovered are not pleasant smelling, though • The most common aromatic ring is benzene which includes 6 Carbons and 6 Hydrogens--it is highly unsaturated 3 Aromatic Compounds and Benzene • Strangely, aromatics do not undergo the same kinds of reactions as alkenes and alkynes • The benzene ring is planar and hexagonal • The three double bonds in benzene are shared equally between the six carbons. We say that the double bonds are delocalized • Benzene is unusually stable and resists most reactants 4 Benzene Structure The structures for benzene can also be written as a single structure where the alternating double bonds are written as a circle within the ring. Benzene structure 5 Aromatic Compounds in Nature and Health Many aromatic compounds are common in nature and in medicine. CHO COOH COOCH3 CH3 CH3 CH3CHCH2 CHCOOH OCH3 OH Aspirin Vanillin Ibuprofen 6 Naming Aromatic Compounds Aromatic compounds are named with benzene as the parent chain. One side group is named in front of the name benzene. CH3 methylbenzene (toluene) Cl chlorobenzene 7 You Try These! • Name the following aromatic compounds. Br CH2CH3 8 Answers to You Try These! • Name the following aromatic compounds. Br Bromobenzene CH2CH3 Ethylbenzene 9 Common Aromatic Compounds You Should Know Nitrobenzene NO2 Phenol OH Toluene CH3 Aniline NH2 10 Naming Aromatic Compounds When two groups are attached to benzene, the ring is numbered to give the lower numbers to the side groups. The prefixes ortho (1,2), meta (1,3) and para (1,4) are also used. Cl CH3 Cl CH3 Cl CH3 ortho-dimethylbenzene meta-dichlorobenzene para-chloromethylbenzene 11 Some Common Names Some substituted benzene rings also use a common name. Then naming with additional more side groups uses the ortho-, meta-, para- system. CH3 CH3 Toluene Cl m-chlorotoluene 12 Some More Common Names OH NO2 OH Phenol p-nitrophenol 13 Other Common Aromatic Compounds You Should Know CH Cl3 Xylene Cl 3 CH OH CH3 Cresol Cl 3 CH 14 You Try These! Select the names for each structure: Cl 1. Chlorocyclohexane 2. Chlorobenzene 3. 1-chlorobenzene CH 3 CH 3 1. Meta-methyltoluene 2. Meta-dimethylbenzene 3. 1,3-dimethylbenzene 15 Answers to You Try These! Select the names for each structure: Cl 2. Chlorobenzene CH 3 1. Meta-methyltoluene 2. Meta-dimethylbenzene 3. 1,3-dimethylbenzene CH 3 16 You Try these! Write the structural formulas for each of the following: A. 1-bromo-3-methylbenzene B. Ortho-chlorotoluene 17 Answers to You Try These! Write the structural formulas for each of the Br following: A. 1-bromo-3-methylbenzene CH3 CH3 B. Ortho-chlorotoluene Cl 18 Homework Assignment Pg. 345-346 12.11 – 12.12 Due Tomorrow! 19