Heat HESS (H) - NordoniaHonorsChemistry
advertisement

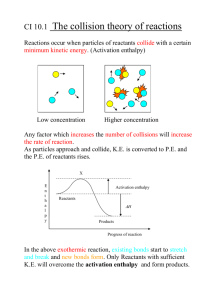
Study of energy relationships in a chemical system We will look at energy in terms of three concepts 1. Temperature 2. Heat 3. Enthalpy (H) 1. 2. 3. Temperature - measurement of avg. KE of particles in an object Heat - heat is the transfer of energy btw 2 objects due to a ∆ temperature Enthalpy ( H) - total energy in a chemical system - we will be concerned with the TRANSFER of enthalpy (∆ H) Temperature ( Celsius and Kelvin) Energy (joules or calories) • Ability of a system to do work or supply ( or produce) heat Heat (CANNOT MEASURE) • We can only measure through changes in temperature Calorie (cal) is the amount of energy needed to raise one gram of water by 1 °C. • kcal = energy needed to raise 1000 g of water 1 °C. • food calories = kcals. Energy Conversion Factors 1 calorie (cal) 1 Calorie (Cal) = = 4.184 joules (J) 1000 calories (cal) 1 kilowatt-hour (kWh) = 3.60 x 106 joules (J) Tro's "Introductory Chemistry", Chapter 3 6 Example • Window in the winter time Energy always flows in the same direction → When does the energy flow stop? Energy is stored in chemicals, found in bonds that hold atoms together As chemical rxn takes place, bonds break, new bonds are created, energy is exchanged Change in energy is change in enthalpy Energy can be released or absorbed Heat releasing (Exothermic) Reactants --> Products + E Heat H = (-) Absorbed (Endothermic) Reactants + E --> Products H = (+) a. How much heat is released when 275 grams of sulfur is burned? b. How much heat is released when 25 mol of sulfur is burned in excess oxygen? c. How much heat is released when 150.0 grams of sulfur dioxide is produced? Some chemical reactions we cannot carry out in a calorimeter they release or absorb tons of energy We must indirectly calculate the change in energy for these reactions We use Hess’ Law Before we discuss Hess’ Law we must first talk about Energy Energy is a state function • This means that the pathway one uses to get from one energy level to another is not important Change in enthalpy for any equation can be calculated without actually carrying out the reaction Just simply add up the H of other related experiments N2(g) + 2O2(g) --> 2NO2 (g) N2(g) + 2O2 (g) --> N2O4 (g) H = 67.8 kJ H = 9.67 kJ