Week-5 - OSU Chemistry
advertisement

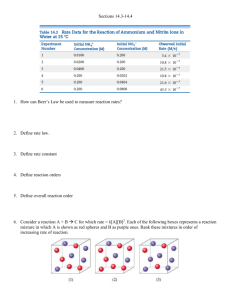
Demo today ??? Colloids • • • • • Hydrophilic and Hydrophobic Colloids Focus on colloids in water. “Water loving” colloids: hydrophilic. “Water hating” colloids: hydrophobic. Molecules arrange themselves so that hydrophobic portions are oriented towards each other. If a large hydrophobic macromolecule (giant molecule) needs to exist in water (e.g. in a biological cell), hydrophobic molecules embed themselves into the macromolecule leaving the hydrophilic ends to interact with water. • • • • • • Hydrophilic and Hydrophobic Colloids…….. Typical hydrophilic groups are polar (containing C-O, O-H, N-H bonds) or charged. Hydrophobic colloids need to be stabilized in water. Adsorption: when something sticks to a surface we say that it is adsorbed. If ions are adsorbed onto the surface of a colloid, the colloids appears hydrophilic and is stabilized in water. Consider a small drop of oil in water. Add to the water sodium stearate. Hydrophilic and Hydrophobic Colloids Hydrophilic and Hydrophobic Colloids • Sodium stearate has a long hydrophobic tail (CH3(CH2)16-) and a small hydrophobic head (-CO2-Na+). • The hydrophobic tail can be absorbed into the oil drop, leaving the hydrophilic head on the surface. • The hydrophilic heads then interact with the water and the oil drop is stabilized in water. Removal of Colloidal Particles • Colloid particles are too small to be separated by physical means (e.g. filtration). • Colloid particles may be coagulated (enlarged) until they can be removed by filtration. • Methods of coagulation: – heating (colloid particles move and are attracted to each other when they collide); – adding an electrolyte (neutralize the surface charges on the colloid particles). – Dialysis: using a semipermeable membranes separate ions from colloidal particles Chapter 14 14.1 14.2 14.3 14.4 14.5 14.5 14.7 Chemical Kinetics Factors that Affect Reaction Rates Reaction Rates Changes of Rate with Time Reaction Rates and Stoichiometry Concentration and Rate Exponents in the Rate Law Units of Rate Constants Using Initial Rates to Determine Rate Laws The Change of Concentration with Time First-Order Reactions Second-Order Reactions Half-Life Temperature and Rate Reaction Mechanisms Catalysis Following the Progress of the Reaction A B C4H9Cl(aq) + H2O (l) C4H9OH (aq) + HCl (aq) Rate [C4 H 9Cl ] [C4 H 9OH ] t t Note the signs! In fact, the instantaneous rate corresponds to d[A]/dt 2 HI(g) H2(g) + I2(g) Consider the reaction It’s convenient to define the rate as 1 [ HI ] [ H 2 ] [ I 2 ] rate 2 t t t And, in general for aA + bB cC + dD 1 [ A] 1 [ B] 1 [C ] 1 [ D] Rate a t b t c t d t Sample exercise 14.2 The decomposition of N2O5 proceeds according to the equation 2 N2O5 (g) 4 NO2 (g) + O2 (g) If the rate of decomposition of of N2O5 at a particular instant in a vessel is 4.2 X 10-7 M/s, what is the rate of appearance of (a) NO2; (b) O2 ? 1 [ N 2O5 ] 1 [ NO2 ] 1 [O2 ] Rate 2 t 4 t 1 t i.e. the rate of disappearance of N2O5 is 4.2 x 10-7 M/s the rate of the reaction is 2.1 x 10-7 M/s the rate of appearance of NO2 is 8.4 x 10-7 M/s and the rate of appearance of O2 is 2.1 x 10-7 M/s 2 N2O5 = 4 NO2 + O2 (g) at T = 45 oC in carbon tetrachloride as a solvent Time min ∆t min [N2O5] mol/L 0 2.33 184 2.08 319 1.91 526 1.67 867 1.35 1198 1.11 1877 0.72 ∆[N2O5] mol/L - ∆[N2O5]/ ∆t mol/L-min 2 N2O5 = 4 NO2 + O2 (g) at T = 45 oC in carbon tetrachloride as a solvent Time min ∆t min 0 [N2O5] mol/L - ∆[N2O5]/ ∆t mol/L-min 0.25 1.36 x 10-3 0.17 1.26 x 10-3 0.24 1.16 x 10-3 0.32 0.94 x 10-3 0.24 0.72 x 10-3 0.39 0.57 x 10-3 2.33 184 184 2.08 135 319 1.91 207 526 1.67 341 867 1.35 331 1198 1.11 679 1877 ∆[N2O5] mol/L 0.72 The information on the previous slide is a bit of a nuisance, since the instantaneous rate keeps changing —and you know how much we like constant values or linear relationships! So let’s try something rather arbitrary at this point. Let’s divide the instantaneous, average rate by [N2O5] and/or [N2O5]2 2 N2O5 = 4 NO2 + O2 (g) at T = 45 oC in carbon tetrachloride as a solvent [N2O5] mol/L [N2O5]av ∆[N2O5] avg mol/L - ∆[N2O5]/ ∆t mol/L-min Avg rate /[N2O5]av Avg rate /[N2O5]av2 2.21 0.25 1.36 x 10-3 6.2 x 10-4 2.8 x 10-4 2.00 0.17 1.26 x 10-3 6.3 x 10-4 3.2 x 10-4 1.79 0.24 1.16 x 10-3 6.5 x 10-4 3.6 x 10-4 1.51 0.32 0.94 x 10-3 6.2 x 10-4 4.1 x 10-4 1.23 0.24 0.72 x 10-3 5.9 x 10-4 4.8 x 10-4 0.92 0.39 0.57 x 10-3 6.2 x 10-4 6.7 x 10-4 2.33 2.08 1.91 1.67 1.35 1.11 0.72 Notice the nice constant value!!! which we’ll call ‘k’ It’s convenient to write this result in symbolic form: Rate = k [N2O5] where the value of k is about 6.2 x 10-4 so that when [N2O5] = 0.221, Rate = (6.2 x 10-4 )(0.221) = 1.37 x 10-4 which is the ‘average rate’ we started with In fact, we really should take into account the 2 in front of the N2O5, in accordance with the rule we developed earlier. This leads us to the general concept of Reaction Order When Rate = k [reactant 1]m [reactant 2]n we say the reaction is m-th order in reactant 1 n-th order in reactant 2 and (m + n)-th order overall. Be careful—because these orders are NOT related necessarily to the stoichiometry of the reaction!!! Other reactions and their observed reaction orders 2 N2O5 = 4 NO2 + O2 (g) Rate = k [N2O5] !!! CHCl3 (g) + Cl2 (g) CCl4 (g) + HCl(g) Rate = k[CHCl3][Cl2]1/2 H2 (g) + I2 (g) 2 HI (g) Rate = k[H2][I2] The order must be determined experimentally!!! We’ll see later that it depends on the Reaction Mechanism, rather than the overall stoichiometry. Be careful: the measurement of the rate will always depend on observations of the reactants or products and involves stoichiometry, but the part on the right, the order, does not depend on the stoichiometry. Let’s explore the results when Rate = k [N2O5] This can be expressed as Rate = - (Δ[N2O5] / Δ t = - d[N2O5] / dt = k [N2O5] or, in general for A products Rate = - Δ[A] / Δt = - d[A] / dt = k [A] rearrangement and integration from time = 0 to t = t gives the result ln[A]t - ln[A]o = - kt or ln [A]t = - kt + ln [A]o or ln ([A]t/[A]o = - kt where [A]o = conc. at t = 0 This is the expression of concentration vs time for a First-Order Reaction Same information in a better format: [ A] d [ A] Rate k[ A] t dt [ A]t [ A]0 t d [ A] k dt 0 [ A] ln[ A]t kt ln[ A]0 To give these forms of the “integrated rate law”: or ln[ A]t ln[ A]0 kt [ A]t or ln kt [ A]0 Consider First-Order Reactions [ A] d [ A] Rate k[ A] t dt [ A]t [ A]0 t d [ A] k dt 0 [ A] ln[ A]t kt ln[ A]0 To give these forms of the “integrated rate law”: or ln[ A]t ln[ A]0 kt [ A]t or ln kt [ A]0 An example of the plots of concentration vs time for a First-Order Reaction The Change of Concentration with Time Half-Life • Half-life is the time taken for the concentration of a reactant to drop to half its original value. • That is, half life, t1/2 is the time taken for [A]0 to reach ½[A]0. • Mathematically, t1 2 ln 1 k 2 0.693 k The Change of Concentration with Time For a First-Order Reaction The identical length of the first and second half-life is a SPECIFIC characteristic of First-Order reactions Consider now Second-Order Reactions [ A] d [ A] Rate k[ A]2 t dt [ A]t [ A]0 t d [ A] k dt 2 0 [ A] 1 1 kt [ A]t [ A]0 Not e the 2! Second-Order Reactions • We can show that the half life 1 t1 2 k A 0 • A reaction also can have rate constant expression of the form rate = k[A][B], i.e., be second order overall, but be first order in A and in B. Recall for 1st order: t1 ln 1 k 2 2 0.693 k And for 2nd order: t1 2 Is this first or second order in the reactant? What is k? 1 k A 0 t1/2 = 1.73 sec (‘2nd half-life’) t1/2 = 0.693/0.4 = 1.73 sec t1/2 = [(0.4)(0.5)] -1 = 5.0 sec (‘2nd half life’) t1/2 = (k[A]0)-1 = [(0.4)(1.0)] -1 = 2.5 sec (‘1st half-life’) MQ-1 122, WI 07: THE MEAN WAS THE MODE WAS 95.00 WITH 10 STUDENTS. THE MEDIAN WAS 112.50 THE STANDARD DEVIATION WAS 29.35 112.00 (64.00%) High: 175 Low 38 N = 472 THE POSSIBLE RANGE WAS FROM 0.00 TO 175.00 THE ACTUAL RANGE WAS FROM 38.00 TO 175.00 (21.7% TO 100.0%) CUTS TAKEN AT 72.88 (APROX. 72.50) AND 26.86 (APROX. 27.50) PERCENTILES. THE UPPER CUT CONTAINED 130 STUDENTS; THE LOWER, 130 STUDENTS. TOTAL NUMBER OF DETECTED ERRORS: 0. DISTRIBUTION OF SCORES 0.00 - 9.99 0 10.00 - 19.99 0 20.00 - 29.99 0 30.00 - 39.99 1 40.00 - 49.99 8 50.00 - 59.99 6 60.00 - 69.99 22 70.00 - 79.99 32 80.00 - 89.99 45 90.00 - 99.99 50 100.00 - 109.99 51 110.00 - 119.99 59 120.00 - 129.99 50 130.00 - 139.99 53 140.00 - 149.99 38 150.00 - 159.99 42 160.00 - 169.99 12 170.00 - 175.00 3 These students are in danger of failing! Please See Me!!! Correct answers: BCDAE CCDDE CADBE BDABD AAADC ACDBA A Chapter 14 14.1 14.2 14.3 14.4 14.5 14.5 14.7 Chemical Kinetics Factors that Affect Reaction Rates Reaction Rates Changes of Rate with Time Reaction Rates and Stoichiometry Concentration and Rate Exponents in the Rate Law Units of Rate Constants Using Initial Rates to Determine Rate Laws The Change of Concentration with Time First-Order Reactions Second-Order Reactions Half-Life Temperature and Rate Reaction Mechanisms Catalysis First Order Reactions d [ A] rate k [ A] dt ln[ A]t kt ln[ A]0 or ln[ A]t ln[ A]0 kt Second Order Reactions d [ A] rate k [ A]2 dt 1 1 kt [ A]t [ A]0 [ A]t or ln kt [ A]0 ln 2 0.693 t 12 k k 1 t1 2 k A0 Example Exercise 14.8 NO2 (g) NO (g) + ½ O2 (g) at 300 oC Page 540 Time/s[NO2] 0.0 50.0 100.0 200.0 300.0 ln[NO2] 0.0100 0.00787 0.00649 0.00481 0.00380 -4.610 -4.845 -5.038 -5.337 -5.573 Is the reaction first or second order in NO2 ? What is the rate constant for the reaction? 1/[NO2] 100 127 154 208 263 Second-Order Processes The decomposition of NO2 at 300°C is described by the equation NO2 (g) NO (g) + 1/2 O2 (g) and yields data comparable to this: Time (s) 0.0 50.0 100.0 200.0 300.0 [NO2], M 0.01000 0.00787 0.00649 0.00481 0.00380 Second-Order Processes • Graphing ln [NO2] vs. t yields: • The plot is not a straight line, so the process is not first-order in [A]. Time (s) 0.0 50.0 [NO2], M 0.01000 0.00787 ln [NO2] −4.610 −4.845 100.0 200.0 300.0 0.00649 0.00481 0.00380 −5.038 −5.337 −5.573 Second-Order Processes • Graphing ln 1/[NO2] vs. t, however, gives this plot. Time (s) 0.0 50.0 100.0 200.0 300.0 [NO2], M 0.01000 0.00787 1/[NO2] 100 127 0.00649 0.00481 0.00380 154 208 263 • Because this is a straight line, the process is secondorder in [A]. Example of Second-Order Plots of conc vs time NO2 (g) NO (g) + ½ O2 (g) at 300 oC (See page 591) Since graph (b) gives a straight line, it is a second-order reaction in NO2 . i.e. rate = k[NO2]2 . And the slope is k, where k = 0.534 M -1 s -1 . Similar problem based on reaction 2 C2F4 C4F8 Similar problem based on reaction C2F4 1/2 C4F8 which we are told is 2nd order in the reactant, with a rate constant of 0.0448 M -1 s-1 or 0.0448 L mol -1 s -1 at 450 K. If the initial concentration is 0.100 M, what will be the concentration after 205 s ? 1/Ct = 1/Co + kt = 1/(0.100 M) + (0.0448 M -1 s-1 )(205 s) = 19.2 M -1 or Ct = 5.21 x 10 -2 M General Order of reaction First Order reactions Second Order reactions Integrated form of each Half lives of each Take out a clean sheet of paper and print on it your name and section number and an indication it is “pop quiz # 2”. Mon Rec, 2:30, 3:30 Wed Rec, 2:30, 3:30 Josh Dettman Brian Culp Venuka Durani Xiangke Chen 115 120 116 119 117 118 Jason Hoy Luyuan Zhang 109 112 110 113 111 113 Take out a clean sheet of paper and print on it your name and section number and an indication it is “pop quiz # 2”. Tue Rec Thur Rec Dan Poole 122 Dan Poole 126 Nick Selner 123 Nick Selner 127 Jim Bowsher 124 Jim Bowsher 125 Yu-Kay Law Nadia Casillas 129 128 Yu-Kay Law 121 A certain reaction has the form A B. At 400 K and [A]0 = 2.8 x 10 -3 M, data for a plot of [A] vs t were collected. It was then found that a plot of 1/[A] vs t yielded a straight line with a slope of 3.60 x 10-2 L∙mol -1 ∙s -1 . a) Write an expression for the rate law. rate = k[A]2 b) Write an expression for the integrated rate law. 1/[A] = 1/[A]0 + kt c) What is the rate constant for the reaction? 3.60 x 10-2 L∙mol -1 ∙s -1 d) What is the half-life for the reaction, as given? 1 1 10 5 3 t1 9 . 9 x 10 2 k [ A]0 (3.60 x102 )( 2.8 x10 3 ) (3.6)( 2.8) A certain reaction has the form A B. At 400 K and [A]0 = 5.6 x 10 -3 M, data for a plot of [A] vs t were collected. It was then found that a plot of 1/[A] vs t yielded a straight line with a slope of 1.80 x 10-2 L∙mol -1 ∙s -1 . a) Write an expression for the rate law. rate = k[A]2 b) Write an expression for the integrated rate law. 1/[A] = 1/[A]0 + kt c) What is the rate constant for the reaction? 1.80 x 10-2 L∙mol -1 ∙s -1 d) What is the half-life for the reaction, as given? 1 1 10 5 3 t1 9 . 9 x 10 2 k [ A]0 (1.80 x102 )(5.6 x10 3 ) (1.8)(5.6) Remember this figure? Let’s use it for another point. The ‘initial rate’ is very useful in determining the order of a reaction. It is the only time when there are no products present! SAMPLE EXERCISE 14.6 Determining a Rate Law from Initial Rate Data The initial rate of a reaction B, and the results are as follows: was measured for several different starting concentrations of A and Using these data, determine (a) the rate law for the reaction, (b) the magnitude of the rate constant, (c) the rate of the reaction when [A] = 0.050 M and [B] = 0.100 M. Solution Analyze: We are given a table of data that relates concentrations of reactants with initial rates of reaction and asked to determine (a) the rate law, (b) the rate constant, and (c) the rate of reaction for a set of concentrations not listed in the table. Plan: (a) We assume that the rate law has the following form: Rate = k[A]m[B]n, so we must use the given data to deduce the reaction orders m and n. We do so by determining how changes in the concentration change the rate. (b) Once we know m and n, we can use the rate law and one of the sets of data to determine the rate constant k. (c) Now that we know both the rate constant and the reaction orders, we can use the rate law with the given concentrations to calculate rate. Solve: (a) As we move from experiment 1 to experiment 2, [A] is held constant and [B] is doubled. Thus, this pair of experiments shows how [B] affects the rate, allowing us to deduce the order of the rate law with respect to B. Because the rate remains the same when [B] is doubled, the concentration of B has no effect on the reaction rate. The rate law is therefore zero order in B (that is, n = 0). SAMPLE EXERCISE 14.6 continued In experiments 1 and 3, [B] is held constant so these data show how [A] affects rate. Holding [B] constant while doubling [A] increases the rate fourfold. This result indicates that rate is proportional to [A] 2 (that is, the reaction is second order in A). Hence, the rate law is This rate law could be reached in a more formal way by taking the ratio of the rates from two experiments: Using the rate law, we have 2n equals 1 under only one condition: We can deduce the value of m in a similar fashion Using the rate law gives SAMPLE EXERCISE 14.6 continued Because 2m = 4, we conclude that (b) Using the rate law and the data from experiment 1, we have (c) Using the rate law from part (a) and the rate constant from part (b), we have Because [B] is not part of the rate law, it is irrelevant to the rate, provided that there is at least some B present to react with A. Check: A good way to check our rate law is to use the concentrations in experiment 2 or 3 and see if we can correctly calculate the rate. Using data from experiment 3, we have Thus, the rate law correctly reproduces the data, giving both the correct number and the correct units for the rate. SAMPLE EXERCISE 14.7 Using the Integrated First-Order Rate Law The decomposition of a certain insecticide in water follows first-order kinetics with a rate constant of 1.45 yr–1 at 12°C. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5.0 10–7 g/cm3. Assume that the average temperature of the lake is 12°C. (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long will it take for the concentration of the insecticide to drop to 3.0 10–7 g/cm3? Solution Analyze: We are given the rate constant for a reaction that obeys first-order kinetics, as well as information about concentrations and times, and asked to calculate how much reactant (insecticide) remains after one year. We must also determine the time interval needed to reach a particular insecticide concentration. Because the exercise gives time in (a) and asks for time in (b), we know that the integrated rate law, Equation 14.13, is required. Plan: (a) We are given k = 1.45 yr–1, t = 1.00 yr, and [insecticide]0 = 5.0 10–7 g/cm3, and so Equation 14.13 can be solved for 1n[insecticide]t. (b) We have k = 1.45yr–1, [insecticide]0 = 5.0 10–7 g/cm3, and [insecticide]t = 3.0 10–7 g/cm3, and so we can solve Equation 14.13 for t. Solve: (a) Substituting the known quantities into Equation 14.13, we have We use the ln function on a calculator to evaluate the second term on the right, giving To obtain [insecticide]t = 1 yr, we use the inverse natural logarithm, or ex, function on the calculator: Note that the concentration units for [A]t and [A]0 must be the same. SAMPLE EXERCISE 14.7 continued (b) Again substituting into Equation 14.13, with [insecticide] t = 3.0 10–7 g/cm3, gives Solving for t gives Check: In part (a) the concentration remaining after 1.00 yr (that is,1.2 10–7 g/cm3) is less than the original concentration (5.0 10–7 g/cm3), as it should be. In (b) the given concentration (3.0 10–7 g/cm3) is greater than that remaining after 1.00 yr, indicating that the time must be less than a year. Thus, t = 0.35 yr is a reasonable answer. PRACTICE EXERCISE The decomposition of dimethyl ether, (CH3)2O, at 510°C is a first-order process with a rate constant of 6.8 10–4s–1: If the initial pressure of (CH3)2O is 135 torr, what is its partial pressure after 1420 s? Answer: 51 torr SAMPLE EXERCISE 14.8 Determining Reaction Order from the Integrated Rate Law The following data were obtained for the gas-phase decomposition of nitrogen dioxide at 300°C, Is the reaction first or second order in NO2? Solution Analyze: We are given the concentrations of a reactant at various times during a reaction and asked to determine whether the reaction is first or second order. Plan: We can plot ln[NO2] and 1/[NO2] against time. One or the other will be linear, indicating whether the reaction is first or second order. SAMPLE EXERCISE 14.8 continued Solve: In order to graph ln[NO2] and 1/[NO2] against time, we will first prepare the following table from the data given: SAMPLE EXERCISE 14.8 continued As Figure 14.8 shows, only the plot of 1/[NO2] versus time is linear. Thus, the reaction obeys a second-order rate law: Rate = k[NO2]2. From the slope of this straight-line graph, we determine that k = 0.543 M–1 s–1 for the disappearance of NO2. PRACTICE EXERCISE Figure 14.8 Kinetic data for decomposition of NO2. The reaction is NO2(g) NO(g) + 1/2O2(g), and the data were collected at 300°C. (a) A plot of [NO2] versus time is not linear, indicating that the reaction is not first order in NO2. (b) A plot of 1/[NO2] versus time is linear, indicating that the reaction is second order in NO2. Consider again the decomposition of NO2 discussed in the Sample Exercise. The reaction is second order in NO 2 with k = 0.543 M–1s–1. If the initial concentration of NO2 in a closed vessel is 0.0500 M, what is the remaining concentration after 0.500 h? Answer: Using Equation 14.14, we find [NO2] = 1.00 10–3 M Using ‘Initial Rates’ to determine order of reaction. Chemical Kinetics (cont) 14.3 14.4 14.5 The Change of Concentration with Time First-Order Reactions Half-Life Second-Order Reactions Temperature and Rate The Collision Model Activation Energy The Orientation Factor The Arrhenius Equation Reaction Mechanisms Elementary Steps Multistep Mechanisms Rate Laws of Elementary Steps Rate Laws of Multistep Mechanisms Mechanisms with and Initial Fast Step Note the DRAMATIC effect of temperature on k Temperature and Rate The Collision Model eg H2 + I2 The Collision Model • The more molecules present, the greater the probability of collision and the faster the rate. • Complication: not all collisions lead to products. In fact, only a small fraction of collisions lead to product. • The higher the temperature, the more energy available to the molecules and the faster the rate. • In order for reaction to occur the reactant molecules must collide in the correct orientation and with enough energy to form products. Activation Energy • Arrhenius: molecules must posses a minimum amount of energy to react. Why? – In order to form products, bonds must be broken in the reactants. – Bond breakage requires energy. • Activation energy, Ea, is the minimum energy required to initiate a chemical reaction. Activation Energy Activation Energy • Consider the rearrangement of acetonitrile: H3C N C H3C N C H3C C N – In H3C-NC, the C-NC bond bends until the C-N bond breaks and the NC portion is perpendicular to the H3C portion. This structure is called the activated complex or transition state. – The energy required for the above twist and break is the activation energy, Ea. – Once the C-N bond is broken, the NC portion can continue to rotate forming a C-CN bond.