Section 14.3
advertisement

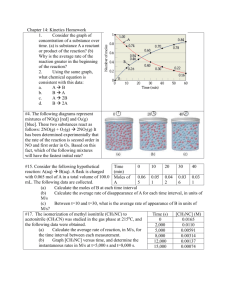
Sections 14.3-14.4 1. How can Beer’s Law be used to measure reaction rates? 2. Define rate law. 3. Define rate constant 4. Define reaction orders 5. Define overall reaction order 6. Consider a reaction A + B C for which rate = k[A][B]2. Each of the following boxes represents a reaction mixture in which A is shown as red spheres and B as purple ones. Rank these mixtures in order of increasing rate of reaction. 7. What are the units of rate 8. (a) What are the overall reaction orders for the reactions described in Equations 14.9 and 14.11? (b) What are the units of the rate constant for the rate law in Equation 14.9? 9. What equation do you use for initial rates to determine rate law 10. The initial rate of a reaction A + B.C was measured for several different starting concentrations of A and B, and the results are as follows: Using these data, determine (a) the rate law for the reaction, (b) the rate constant, (c) the rate of the reaction when [A] = 0.050M and 3B4 = 0.100 M. 11. Define first-order reaction 12. What else is the first order reaction known as? 13. The decomposition of a certain insecticide in water at 12 °C follows first-order kinetics with a rate constant of 1.45 yr-1. A quantity of this insecticide is washed into a lake on June 1, leading to a concentration of 5.0 * 10-7 g/cm3. Assume that the temperature of the lake is constant (so that there are no effects of temperature variation on the rate). (a) What is the concentration of the insecticide on June 1 of the following year? (b) How long will it take for the insecticide concentration to decrease to 3.0 * 10-7 g/cm3? 14. Define second-order reaction 15. The following data were obtained for the gas-phase decomposition of nitrogen dioxide at 300 °C, NO2(g) NO(g) + 12 O2(g). Is the reaction first or second order in NO2? 16. Define Zero-Order Reaction 17. Define half-life 18. The reaction of C4H9Cl with water is a first-order reaction. (a) Use Figure 14.4 to estimate the halflife for this reaction. (b) Use the half-life from (a) to calculate the rate constant. 19. Define equation 14.17 20. A reaction A + B C obeys the following rate law: Rate = k[B]2. (a) If [A] is doubled, how will the rate change? Will the rate constant change? (b) What are the reaction orders for A and B? What is the overall reaction order? (c) What are the units of the rate constant? 21. The decomposition reaction of N2O5 in carbon tetrachloride is 2 N2O5 4 NO2 + O2. The rate law is first order in N2O5. At 64 °C the rate constant is 4.82 * 10-3 s-1. (a) Write the rate law for the reaction. (b) What is the rate of reaction when (N2O5) = 0.0240 M? (c) What happens to the rate when the concentration of N2O5 is doubled to 0.0480 M? (d) What happens to the rate when the concentration of N2O5 is halved to 0.0120 M? 22. Consider the following reaction: CH3Br(aq) + OH-(aq) CH3OH(aq) + Br-(aq) The rate law for this reaction is first order in CH3Br and first order in OH- . When (CH3Br) is 5.0 * 10-3 M and 3OH- 4 is 0.050 M, the reaction rate at 298 K is 0.0432 M/s. (a) What is the value of the rate constant? (b) What are the units of the rate constant? (c) What would happen to the rate if the concentration of OH- were tripled? (d) What would happen to the rate if the concentration of both reactants were tripled? 23. The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way: OCl- + I- OI- + Cl- . This rapid reaction gives the following rate data: (a) Write the rate law for this reaction. (b) Calculate the rate constant with proper units. (c) Calculate the rate when [OCl-] = 2.0 * 10-3 M and [I -] = 5.0 * 10-4 M. 24. The following data were measured for the reaction BF3(g) + NH3(g) F3BNH3(g): (a) What is the rate law for the reaction? (b) What is the overall order of the reaction? (c) Calculate the rate constant with proper units? (d) What is the rate when [BF3] = 0.100 M and[NH3] = 0.500 M? 25. Consider the gas-phase reaction between nitric oxide and bromine at 273 °C: 2 NO(g) + Br2(g) 2 NOBr(g). The following data for the initial rate of appearance of NOBr were obtained: (a) Determine the rate law. (b) Calculate the average value of the rate constant for the appearance of NOBr from the four data sets. (c) How is the rate of appearance of NOBr related to the rate of disappearance of Br2? (d) What is the rate of disappearance of Br2 when [NO] = 0.075 M and [Br2] = 0.25 M ? 26. (a) Define the following symbols that are encountered in rate equations for the generic reaction A B: [A]0, t1/2 [A]t, k. (b) What quantity, when graphed versus time, will yield a straight line for a first-order reaction? (c) How can you calculate the rate constant for a first-order reaction from the graph you made in part (b)? 27. (a) The gas-phase decomposition of SO2Cl2, SO2Cl2(g) SO2(g) + Cl2(g), is first order in SO2Cl2. At 600 K the halflife for this process is 2.3 * 105 s. What is the rate constant at this temperature? (b) At 320 °C the rate constant is 2.2 * 10-5 s-1. What is the half-life at this temperature? 28. As described in Exercise 14.41, the decomposition of sulfuryl chloride (O2Cl2) is a first-order process. The rate constant for the decomposition at 660 K is 4.5 * 10-2 s-1. (a) If we begin with an initial SO2Cl2 pressure of 450 torr, what is the partial pressure of this substance after 60 s? (b) At what time will the partial pressure of SO2Cl2 decline to one-tenth its initial value? 29. The reaction SO2Cl2(g) SO2(g) + Cl2(g) is first order in SO2Cl2. Using the following kinetic data, determine the magnitude and units of the first-order rate constant: 30. Consider the data presented in Exercise 14.19. (a) By using appropriate graphs, determine whether the reaction is first order or second order. (b) What is the rate constant for the reaction? (c) What is the half-life for the reaction? The gas-phase decomposition of NO2, 2 NO2(g) 2 NO(g) + O2(g), is studied at 383 °C, giving the following data: (a) Is the reaction first order or second order with respect to the concentration of NO2? (b) What is the rate constant? (c) Predict the reaction rates at the beginning of the reaction for initial concentrations of 0.200 M, 0.100 M, and 0.050 M NO2.