The United States' dependence on foreign oil reserves has started to
advertisement

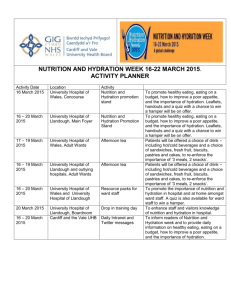
The United States’ dependence on foreign oil reserves has started to affect the U.S. economy. Gasoline prices are rising which, in hand, causes the price of other goods to go up because of the increased cost of associated transportation. While the U.S. economy has suffered, Exxon Mobile reported a record $45.2 billion profit in 2008. (Porretto, 2009) This has lead the U.S. to search for potential answers to the energy crisis. One of the answers, to this problem, is finding a renewable gasoline source. The renewable source is ethanol. Ethanol can be synthesized from a variety of sources; with the fermentation of corn being the major source. Another method is the hydration of ethylene into ethanol. This paper will discuss the mechanism of hydration. Hydration of alkenes, double bonded hydrocarbon chains, follows Markovnikov’s Rule. The rule states that alkenes, in the presence of water, will have the hydroxyl (-OH) group bond to the carbon with the most other carbons bonded to it. The hydrogen, from the water molecule, will then bond to the carbon with the most hydrogen atoms already bonded. (Markovnikov's Rule) Before the mechanism of hydration of alkenes is discussed, the conditions of the reaction must be determined. The reaction of ethene to form ethanol proceeds in the presence of steam and solid silicone dioxide coated with phosphoric acid at 300ºC and high pressure (60-70atms). (Clark, The mechanism for the hydration of ethene, 2002) The first step is the pi, double, bond breaking due to the strong affinity of the positively charged dissociated hydrogen atom from the catalyst. This causes the formation of a carbonium ion which reacts in the next step. (Clark, The mechanism for the hydration of ethene, 2002) Figure 1. Step 1 of hydration. Note: Copyright Jim Clark 2002. The second step has the newly formed carbonium ion attract the lone pair of electrons in a water molecule and form a bond with them. (Clark, The mechanism for the hydration of ethene, 2002) Figure 2. Step 2 of hydration. Note: Copyright Jim Clark 2002. The final step is the reformation of a phosphoric acid molecule by stealing a hydrogen atom from the carbon-oxygen bond. (Clark, The mechanism for the hydration of ethene, 2002) Figure 3. Step 3 of hydration. Note: Copyright Jim Clark 2002. The entire hydration mechanism of ethene is reversible and only yields 5% conversion. To compensate for this, unreacted ethene is recycled through the reactor to yield an overall conversion of 95%. (Clark, The Direct Hydration of Alkenes, 2003) Ethene is recycled by having the effluent (exiting) stream of the reactor cooled until ethanol liquifies. The gaseous ethene is then sent to the entrance of the reactor for another run through it. Water also liquifies, so fractional distillation is needed to separate the water and ethanol. (Clark, The Direct Hydration of Alkenes, 2003) Figure 4. Manufacturing ethanol. Note: Copyright Jim Clark 2003. Hydration of ethene, fermentation of corn, and other reaction mechanisms have the potential to reduce the U.S.’ dependence on foreign oil reserves. The economy, in the future, will be less affected by the fluxuations in oil prices because the internal combustion engine, in automobiles, will be able to run on a fuel source found right here in the U.S.A. We have the potential to stop the rediculous profits made by oil companies at the expense of everyday American citizens. Clark, J. (2003). The Direct Hydration of Alkenes. Retrieved June 8, 2009, from Chemguide.co.uk: http://www.chemguide.co.uk/organicprops/alkenes/hydration.html Clark, J. (2002). The mechanism for the hydration of ethene. Retrieved June 8, 2009, from The Mechanism For the Acid Catalysed Hydration of Ethene: http://www.chemguide.co.uk/physical/catalysis/hydrate.html Markovnikov's Rule. (n.d.). Retrieved June 8, 2009, from Organic-Chemistry.org: http://www.organicchemistry.org/namedreactions/markovnikovs-rule.shtm Porretto, J. (2009, January 30). Exxon Mobil Reports Record $45.2 Billion Profit For 2008. Retrieved June 8, 2009, from The Huffington Post Web site: http://www.huffingtonpost.com/2009/01/30/exxon-mobilreports-recor_n_162468.html