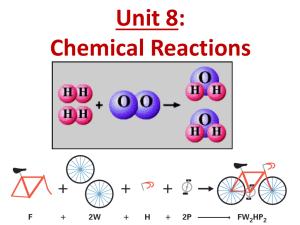
balanced chemical equation
advertisement

(2.3) Balancing Chemical Equations (p150) Conservation of Mass As we saw in class, the mass of the reactants must equal the mass of the products in a chemical reaction. To show that a reaction is balanced, we must write a balanced chemical equation. In a balanced chemical equation, the number of atoms on each side of the equation is the same. How To Write a Balanced Chemical Equation 1. The chemical formula for a substance CANNOT be changed! ex. CO2 molecules must stay as CO2 How To Write a Balanced Chemical Equation 2. From the word equation, write the chemical equation using the correct chemical formula for all the substances involved. ex. The combustion of ethanol (C2H6OH) ethanol+ oxygen carbon dioxide + water C2H6OH + O2 CO2 + H2O How To Write a Balanced Chemical Equation 3. Count the number of atoms in both the reactants and products. 4. Multiply each formula by a coefficient to balance the number of each kind of atom in both the reactants and products. THE RAP SHEET The RAP sheet is a methodology used to help balance chemical equations. It helps keep track of the number and kinds of atoms in a chemical equation. C2H5OH + O2 CO2 + H2O Add coefficient 2 CO2 Add coefficient 3 to H2O Add coefficient 3 to O2 R A P Reactants Atoms Products 7 2 C 1 6 H 2 3 O 3 2 6 5 7 TRY THESE Worksheets 2.3 (D) and 2.3 (E) (2.3) Balancing Chemical Equations (p150) ASSIGNMENTS: Workbook p56 p60-63