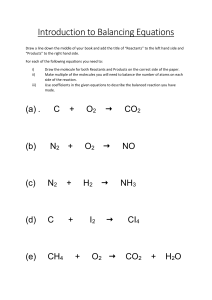
Practice Problems/ Quiz 2 1. 4Al + 3O2 → xAl2O3. Find 'x' A. 3 B. 2 C. 4 D. 5 2. C3H8 + 5O2 → 3CO2 + xH2O.Find 'x'. A. 3 B. 4 C. 5 D. 6 3. Balance the following chemical equation: a) CH4 + O2 → CO2 + H2O Answer: CH4 + 2O2 → CO2 + 2H2O b) CaCO3 + HCl → CaCl2 + CO2 + H2O Answer: CaCO3 + 2HCl → CaCl2 + CO2 + H2O 4. Which information is not conveyed by a balanced chemical equation? A. Physical states of reactants and products B. Symbols and formulae of all the substances involved in a particular reaction C. Number of atoms/molecules of the reactants and products formed D. Whether a particular reaction is actually feasible or not 5. A balanced chemical equation is in accordance with A. Avogadro's law B. Law of multiple proportion C. Law of conservation of mass D. Law of gaseous volumes 6. Workout the formula for the following compounds a) Magnesium fluoride MgF2 b) Sodium carbonate Na(OH)2 c) Magnesium Sulfate MgSO4 d) Sodium hydrogen carbonate NaHCO3 7. Write the balanced chemical equation for the following reaction. Include state symbols. Calcium carbonate decomposes to give calcium oxide and carbon dioxide. Answer: CaCO3(s) → CaO(s) + CO2(g) The reaction is already balanced.