week 4: diels-alder reaction
advertisement

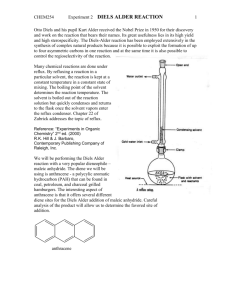
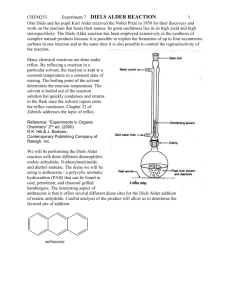
WEEK 4: DIELS-ALDER REACTION PURPOSE: This experiment will introduce the student to an important cycloaddition reaction. The Diels Alder reaction is reversible and the student will need to consider methods to increase the yield, if possible. IMPORTANT REACTIONS: Diene Dienophile Adduct H O H H O O H O -phellandrene (M. Mass 136 bp 175-176oC) Maleic anhydride (M. Mass 98 mp 54-56o) O O endo-7-isopropyl-4-methylbicyclo[2.2.2] oct-4-ene-1,2-dioic anhydride (M. Mass 234, mp 126-127oC) BACKGROUND INFORMATION: The Diels Alder reaction is an example of a cycloaddition reaction discovered by two German chemists (Otto Diels and Kurt Alder) in the 1950’s. This reaction is an example of a concerted reaction in which bonds are being made as other bonds are being broken. Effective overlap of all bonding orbitals is essential for the reaction to occur which leads to a specific stereochemistry in the products. In this reaction, the endo isomer is formed. The Diels Alder reaction is an example of a 2-4 cycloaddition reaction in which an alkene adds in a 1,4 addition to a diene. Neither Diels nor Alder fully understood the generality or significance of cycloaddition reactions. This was left for Roald Hofmann (now at Cornell University) and R. B. Woodward from Harvard University to discover in the late 1960’s. The Woodward Hofmann rules for cycloaddition reactions (which are beyond the scope of this discussion) were perhaps one of the major discoveries in organic synthesis in the 20th century. In early work with this reaction, results were mixed and yields varied quite a bit. Later it was found that this reaction is reversible. If one considers LeChatelier’s rule, it might be possible to shift the equilibrium in the desired direction. In the experiment that will be done here, alphaphellandrene will react with maleic anhydride. The alphaphellandrene is a natural product derived from cedar wood and has a characteristic cedar wood odor. It is not 100% pure so the directions call for an added amount to be used to compensate for the impurity. Maleic anhydride is a white solid. It can hydrolyze with water to give maleic acid so water should be avoided if possible. With prolonged exposure to moisture, the anhydride group in the product may convert to the diacid which will have different properties. The final bicyclic product is usually obtained without difficulty. EXPERIMENTAL PROCEDURE: This reaction must be done in a fume cupboard. Place maleic anhydride (0.527 g, 5.4 mmoles) and -phellandrene (1 mL, 85 % pure, 5.4 mmoles) into a 25 mL round bottomed flask and attach a water-cooled condenser. Add anhydrous acetone (3 mL) through the top of the condenser and swirl the reaction mixture. The reaction is exothermic and a yellow colour should appear at this point. Heat the reaction under reflux for 1 hour using a steam bath. After this time, carefully remove the condenser and allow the acetone solvent to evaporate off to approximately half of its original volume. Allow the reaction to cool to room temperature, then cool the reaction flask in an ice bath to induce crystallization of your Diels-Alder adduct (scratching with a glass rod may be required). Filter off your product using a Hirsch funnel, washing the flask with a small amount of icecold acetone. Dry and weigh the final product, record the melting point and determine the percent yield. If required, (i.e. if the melting point of the product is much lower than expected) recrystallize the product using the minimum amount of warm methanol (be very careful to not overheat as methanolysis of the anhydride group could occur). IMPORTANT INFORMATION ABOUT THE REPORT: The report for this experiment will follow the usual format. Be sure the percent yield calculation is carefully done. Also, record the melting point range of the final product and compare that melting point to the reported melting point of the product given above. Using these data, discuss the relative success on the experiment. END OF EXPERIMENT. © 2007 STEPHEN ANDERSON AND ROBERT SHINE