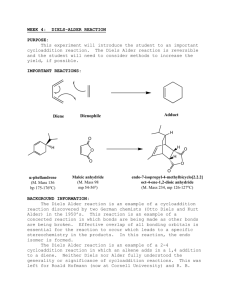
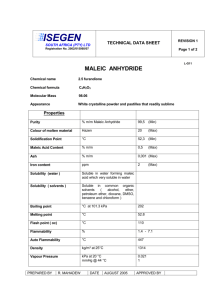
Diels-Alder Reaction: Alpha-Phellandrene & Maleic Anhydride
advertisement

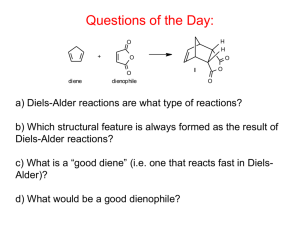
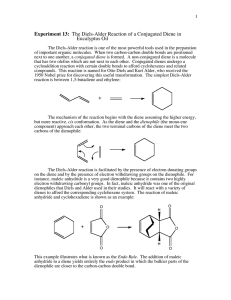
Diels Alder Read pages 412-417. Run the Diels Alder condensation reaction between alpha-phellandrene and maleic anhydride according to the directions below. Obtain IR (KBr pellet) spectrum of the product. Write up a pre-lab for the condensation of α-phellandrene (2-methyl-5-isopropyl1,3-cyclohexadiene) and maleic anhydride. You won't find the product in the CRC. The α-phellandrene that we have is not pure, it only contains 70% α-phellandrene by weight. You will need to figure how much of the impure compound to weigh out that will contain 0.050 mole of the α-phellandrene. In a 100-mL round-bottom flask put 0.050 mole of maleic anhydride and the weight of impure α-phellandrene that contains 0.050 mole. Add 25 mL of ethyl acetate, attach a reflux condenser, and heat on a hot water wath for one hour. Cool in an icewater bath and then suction filter. Recrystallize the product from ethyl acetate, vacuum filter, let air dry, weigh, package, and obtain the IR spectrum (KBr method). answer the following questions: 1) Why is the endo product usually preferred in Diels-Alder condensations? 2) In your product, which way is the isopropyl group pointed? Explain. 3) The product of your synthesis has three chiral centers. Draw the product and label each chiral center with and asterisk (*). How many stereosiomers are theoretically possible? Only one stereoisomer is actually formed in this reaction, explain. 4) Predict the products of the following: a) b) c) d) e) 1,3-butadiene + 2-butyne 1,3-cyclopentadiene + cis-2-butene 1,3-butadiene + dimethyl maleate(methyl ester of maleic acid) (2 mol)1,3-cyclopentadiene + p-benzoquinone dicylopentadiene + heat (retro Diels-Alder) 5) Explain why the diene must be in the sigma-cis conformation in order to undergo a Diels-Alder reaction.