TRANSITION METALS
advertisement
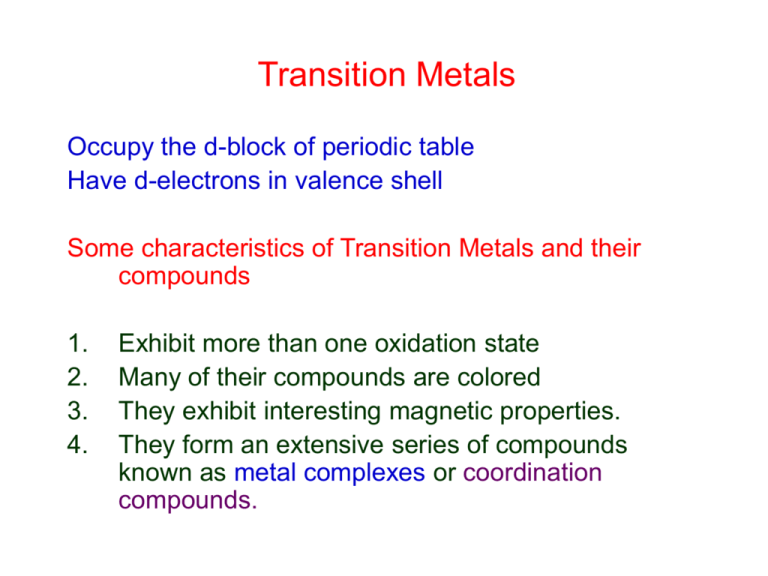
Transition Metals Occupy the d-block of periodic table Have d-electrons in valence shell Some characteristics of Transition Metals and their compounds 1. 2. 3. 4. Exhibit more than one oxidation state Many of their compounds are colored They exhibit interesting magnetic properties. They form an extensive series of compounds known as metal complexes or coordination compounds. Transition Metals Exhibit more than one oxidation state e.g., Reduction of V5+ by metallic Zn VO2(H2O)4+ yellow-orange VO(H2O)52+ blue V(H2O)63+ green V(H2O)62+ violet Many of their compounds are colored ELECTRON CONFIGURATIONS 3d elements: Sc Zn Ar 3s23p6 K [Ar]4s1 Ca [Ar]4s2 Sc [Ar]3d14s2 Ti [Ar]3d24s2 . . . . . . Zn [Ar]3d104s2 Note: 4s is filled before 3d, but when oxidized, 4s electrons are lost before 3d. Ti Ti2+ Ti3+ Ti4+ Ti5+ [Ar]3d24s2 [Ar]3d24s0 [Ar]3d14s0 [Ar]3d04s0 does not exist! Transition Metals TRANSITION METALS: ScMn Oxidation States: Highest oxidation states of Sc, Ti, V, Cr, Mn = number of valence (4s + 3d) electrons. Sc [Ar]3d14s2 Mn [Ar]3d54s2 Sc3+ [Ar] Mn7+[Ar] maximum maximum Trend from Sc Mn: The max. oxidation state becomes increasingly unstable. Sc3+, Ti4+ are stable (maximum oxidation states). Sc2O3 Stable oxide. Mn7+ Exists but is easily reduced. MnO4Strong oxidizing agent. Magnetic Properties Diamagnetic: unaffected by a magnetic field no unpaired electrons Paramagnetic: influenced by a magnetic field unpaired electrons Transition metals and their compounds are often paramagnetic Have unpaired d-electrons Eg. Ti2+ Mn2+ TRANSITION METAL IONS Transition metal ions are Lewis acids they accept electron pairs. Ligands are Lewis bases molecules or ions which donate electron pairs. Ligands bonded to metal ions metal complexes or coordination compounds. Coordination number: number of electron donor atoms attached to the metal. Chelates are ligands possessing two or more donor atoms. COORDINATION COMPOUNDS • Metals-Lewis acids • Ligands -Lewis bases. Ligand molecules have lone pair electrons. – Anions F, Cl, Br, CN, SCN, NO2, etc. – Neutral ligands: NH3, H2O, CO • mono-dentate -(single claw to hold onto metal d orbital) Ex. :NH3, H-:O:-H , CH3-:O:-H • Bi-dentate -(has 2 claws to hold onto metal d orbitals). Has 2 or more functional groups on ligands that have lone pairs Example :NH2-CH2-CH2-H2N: (= en or ethylenediammine) COORDINATION COMPOUNDS Coordination # = 4 Tetrahedral, e.g. [Zn(NH3)4]2+ Square Planar, e.g. [Ni(CN)4]2 Cl Square Planar, e.g. [PtCl3(C2H4)] H H C Cl Pt C Cl H H COORDINATION COMPOUNDS Coordination # = 6 F Octahedral, e.g. [CoF6]3- F F Co F F F Octahedral, e.g. [Co(en)3]3+ N N N Co N N N IMPORTANT CHELATING LIGANDS Porphine O HOCCH2 HOCCH2 O : NCH2CH2N : EDTA O CH2COH CH2COH O CHELATE EFFECT Chelating ligands form more stable compounds. [Ni(H2O)6]2+ + 6NH3 [Ni(NH3)6]2+ + 6H2O Kf = 4x108 [Ni(H2O)6]2+ + 3en [Ni(en)3]2+ + 6H2O Kf = 2x1018 CHELATE EFFECT IS AN ENTROPY EFFECT Cd2+ + 4CH3NH2 [Cd(CH3NH2)4]2+ G° = 37.2kJ H° = 57.3kJ S° = 67.3J/K Cd2+ + 2en [Cd(en)2]2+ G° = 60.7kJ H° = 56.5kJ S° = +14.1J/K PROPERTIES OF TRANSITION METALS Transition Metal Complexes have different properties – • color (all except Zn or Sc3+ white compounds) • solubility-depends on complex reduction potential – lower than free ions Ag+(aq) + e Ag(s) E°1/2= +0.80V [Ag(CN)2](aq) + e Ag(s)+ 2CN(aq) E°1/2 = 0.31V FCo3+ (3d6) Co3+ FF- F F- Co FF- F F F F F CRYSTAL FIELD SPLITTING d-electron energy dz2 dx2-y2 dxy dyz dxz = crystal field splitting energy Spectrochemical series: CN > NO2 > en > NH3 > H2O > OH- > F > Cl decreasing SPECTROCHEMICAL SERIES UV IR Absorbed light CNCO Strong field NO2ligands en NH3 H2O Oxalate OHFWeak field SCN ligands ClBrIColor seen is complementary to absorbed color COLOR WHEEL RED VIOLET ORANGE BLUE YELLOW GREEN CRYSTAL FIELD SPLITTING ENERGY depends on 1. Metal 2. Oxidation state 3. Ligands P = spin pairing energy P does not depend on the ligands P < Low Spin Complex P > High Spin Complex SPIN PAIRING OCTAHEDRAL COMPLEXES E CoF63High spin Paramagnetic Co(CN)63Low spin (spin paired) diamagnetic USES OF TRANSITION METALS Ti Lighter and stronger than steel. Ti and its alloys are used in jet engines, planes, and in special high temp applications, e.g. in the reentry shield on the Apollo capsules. TiO2 is a white pigment in all white paints. V Vanadium steel (Fe/V alloy) is the toughest steel known. It is used in car springs. V2O5 is a catalyst used in sulfuric acid production. Cr Stainless Steel = 73% Fe,18% Cr, 8% Ni, 1% C Chromium is electroplated to make shiny metal parts. Mn Mn steel (Fe/Mn alloy) is very tough and can withstand shock and abrasion – used in bulldozer blades and armor plates on warships. CHROMIUM OXIDES Cr(III) Oxide, Cr2O3 Abrasive, Refractory Semiconductor, Green pigment Amphoteric Cr(IV) Oxide, CrO2 Recording tape (magnetic material) Cr(VI) Oxide, CrO3 Red Chrome plating, corrosion inhibitor Na2Cr2O7 Tanning, metal corrosion inhibitor