Chapter 22-Newest-CD
advertisement

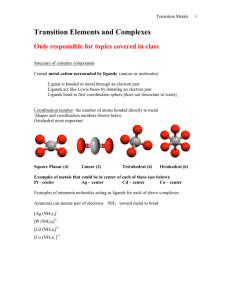
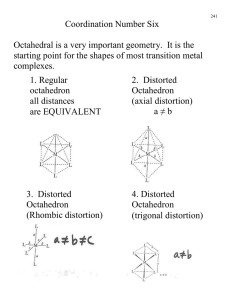
Chapter 23: The Transition Elements and Their Coordination Compounds 23.1 An Overview of Transition Element Properties 23.2 The Inner Transition Elements 23.3 Highlights of Selected Transition Metals 23.4 Coordination Compounds 23.5 Theoretical Basis for the Bonding and Properties of Complexes Fig. 23.1 Orbital Occupancy of the Period 4 Metals–I Element Sc Partial Orbital Diagram 4s 3d Unpaired Electrons 4p 1 Ti 2 V 3 Cr 6 Mn 5 Table 23.1 (p. 1003) Orbital Occupancy of the Period 4 Metals–II Element Fe Partial Orbital Diagram 4s 3d Unpaired Electrons 4p 4 Co 3 Ni 2 Cu 1 Zn 0 Table 23.1 (p. 1003) Fig. 23.3 Fig. 23.4 Aqueous Oxoanions of V, Cr, and Mn in their Highest Oxidation States Fig 23.5 Oxidation States and d-Orbital Occupancy of the Period 4 Transition Metals Oxidation State 0 3B (3) Sc 4B (4) Ti 0 (d1) 0 (d2) +1 +2 +3 +4 +5 +6 +7 +3 (d0) 5B (5) V 6B (6) Cr 0 0 (d3) (d5) +1 +1 (d3) (d5) +2 +2 +2 (d2) (d3) (d4) +3 +3 +3 (d1) (d2) (d3) +4 +4 +4 (d0) (d1) (d2) +5 +5 (d0) (d1) +6 (d0) 7B (7) Mn 8B (8) Fe 8B (9) Co 0 0 0 (d5) (d6) (d7) +1 +1 (d5) (d7) +2 +2 +2 (d5) (d6) (d7) +3 +3 +3 (d4) (d5) (d6) +4 +4 +4 (d3) (d4 ) (d5) +5 +5 (d2) (d4) +6 +6 (d1) (d2) +7 (d0) 8B (10) Ni 1B (11) Cu 2B (12) Zn 0 0 0 (d 8) (d10) (d10) +1 +1 (d8) (d10) +2 +2 +2 (d8) (d9) (d10) +3 +3 (d7) (d8) +4 (d6) Table 23.2 (p. 1006) Standard Electrode Potentials of Period 4 M2+ Ions E0 (V) Half-Reaction Ti2+(aq) + 2 eV2+(aq) + 2 eCr2+(aq) + 2 eMn2+(aq) + 2 eFe2+(aq) + 2 eCo2+(aq) + 2 eNi2+(aq) + 2 eCu2+(aq) + 2 eZn2+(aq) + 2 e- Table 23.3 (p. 1007) Ti(s) V(s) Cr(s) Mn(s) Fe(s) Co(s) Ni(s) Cu(s) Zn(s) -1.63 -1.19 -0.91 -1.18 -0.44 -0.28 -0.25 0.34 -0.76 Colors of Representative Compounds of the Period 4 Transition Metals b a d c f e h g j i a = Scandium oxide f = Potassium ferricyanide b = Titanium(IV) oxide g = Cobalt(II) chloride hexahydrate c = Vanadyl sulfate dihydrate h = Nickel(II) nitrate hexahydrate d = Sodium chromate i = Copper(II) sulfate pentahydrate e = Manganese(II) chloride tetrahydrate j = Zinc sulfate heptahydrate Fig. 23.6 (p. 1012) Coordination Compounds: Complexes • Lewis acids are electron pair acceptors. • Coordination compounds are metal compounds formed by Lewis acid-base interactions. • Complexes: Have a metal ion (can be zero oxidation state) bonded to a number of ligands. Complex ions are charged. Example, [Ag(NH3)2]+. • Ligands are Lewis bases. • Coordination number: the number of ligands attached to the metal. Coordination Numbers and Geometry • The most common coordination numbers are 4 and 6. • Some metal ions have a constant coordination number (e.g. Cr3+ and Co3+ have a coordination number of 6). • The size of the ligand affects the coordination number (e.g. [FeF6]3- forms but only [FeCl4]- is stable). • The amount of charge transferred from ligand to metal affects coordination number (e.g. [Ni(NH3)6]2+ is stable but only [Ni(CN)4]2- is stable). • Four coordinate complexes are either tetrahedral or square planar (commonly seen for d 8 metal ions). • Six coordinate complexes are octahedral. (Square brackets enclose the metal ion and ligands.) Fig. 23.9 (p. 1017) Ligands • Monodentate ligands bind through one donor atom only. – Therefore they occupy only one coordination site. • Polydentate ligands (or chelating agents) bind through more than one donor atom per ligand. – Example, ethylenediamine (en), H2NCH2CH2NH2. • The octahedral [Co(en)3]3+ is a typical en complex. • Chelate effect: More stable complexes are formed with chelating agents than with the equivalent number of monodentate ligands. (p. 1018) Nomenclature • Rules: – For salts, name the cation before the anion. Example in [Co(NH3)5Cl]Cl2 we name [Co(NH3)5Cl]2+ before Cl-. – Within a complex ion, the ligands are named (in alphabetical order) before the metal. Example [Co(NH3)5Cl]2+ is tetraamminechlorocobalt(II). Note the tetra portion is an indication of the number of NH3 groups and is therefore not considered in the alphabetizing of the ligands. – Anionic ligands end in -o and neutral ligands are simply the name of the molecule. Exceptions: H2O (aqua) and NH3 (ammine). Nomenclature • Rules: – Greek prefixes are used to indicate number of ligands (di-, tri-, tetra-, penta-, and hexa-). Exception: if the ligand name has a Greek prefix already. Then enclose the ligand name in parentheses and use bis-, tris-, tetrakis-, pentakis-, and hexakis. • Example [Co(en)3]Cl3 is tris(ethylenediamine)cobalt(III) chloride. – If the complex is an anion, the name ends in -ate. – Oxidation state of the metal is given in Roman numerals in parenthesis at the end of the complex name. Names of Some Neutral and Anionic Ligands Name A. Neutral Aqua Ammine Carbonyl Nitrosyl B. Anionic Fluoro Chloro Bromo Iodo Hydroxo Cyano Table 23.8 (p. 1019) Formula H2O NH3 CO NO FClBrIOHCN- Names of Some Metal Ions in Complex Anions Metal Name in Anion Iron Ferrate Copper Cuprate Lead Plumbate Silver Argentate Gold Aurate Tin Stannate Table 23.9 (p. 1019) Some Coordination Compounds of Cobalt Studied by Werner Werner’s Data* Total Free Ions Cl- Traditional Formula . CoCl . 5 NH CoCl . 4 NH CoCl . 3 NH CoCl3 6 NH3 Modern Formula Charge of Complex Ion 4 3 [Co(NH3)6]Cl3 3+ 3 3 3 2 [Co(NH3)5Cl]Cl2 2+ 3 3 2 1 [Co(NH3)4Cl2]Cl 1+ 3 3 0 0 [Co(NH3)3Cl3] --- Table 23.10 (p. 1020)