synthetic organic polymers
advertisement

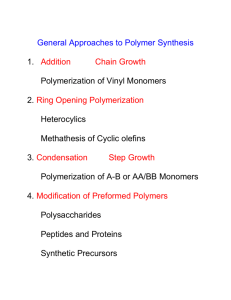
SYNTHETIC ORGANIC POLYMERS Convenor: Dr. Fawaz Aldabbagh Polymers are large molecules made up of repeating units called Monomers The synthetic process is Polymerization. E.g. Polymerization H2C CH2 CH2 CH2 Monomer Polymer O Monomer n Polymerization CH2 CH2 O n Polymer Note – define repeating unit in terms of monomer structure Degree of Polymerization is the number of monomer units in a Polymer However, for synthetic polymers it is more accurate to state average degree of polymerization (DP ¯) A polymer prepared from a single monomer is a homopolymer If two or more monomers are employed, the product is a copolymer Linear polymer has no branching Graft copolymer is an example of a branched network Two main classifications of Polymerization Addition reaction or Chain growth Molecular weight increases by successively adding monomers to a reactive polymer chain end resulting in high molecular weights at low conversions. STEP reaction or growth Polymers are formed by linking monomer molecules to form dimers, trimers and higher species in a step-wise fashion. The most abundant species react, and thus high molecular weight formed only beyond 99% conversion. Polymerization Conversion (p) P = M0 - Mt M0 M0 = initial number of monomer molecules Mt = Number of monomer molecules at time t Ionic Chain (addition)-Growth Polymerization The choice of ionic procedure depends greatly on the electronic nature of the monomers to be polymerized Vinyl monomers with electron-withdrawing groups CN CN CO2R CO2R CO2R Anionic Polymerization Vinyl monomers with electron-donating groups N OR SR Cationic Polymerization Monomers and reagents should be scrupulously purified; water and oxygen should be removed. Polymerizations carried out at very low temperatures Anionic Polymerizations Initiators include alkyl lithiums and sodium amide Cationic Polymerization -- the formed carbocation must be quite stable + Stable tertiary carbocation H+ OR H+ + OR stable oxonium ion E.g. proton initiates polymerization of isobutane (2-methylpropene) BF3/H2O n Adhesive, sealant, insulating oil, lubricating oil Reactions of water with reactive carbanions and carbocations H _ CN CN O H H acid CN CN CN CN n + _ OH Note – viable substrates for anionic polymerizations do not have -protons H H O H base H + OMe OMe OMe OMe OMe n + H3O+ OMe n n Chain Reaction: Free Radical Polymerization RO Initiation 2 RO OR RO + RO Ph Propagation Ph RO RO Ph n Ph Random Termination n Ph Ph Dead chains Conventional Radical Polymerization Advantages – 1/wide range of vinyl monomers polymerizable 2/can be carried out in bulk, water, organic solvents and other solvents 3/no rigorous purification or drying of reagents required Conditions: Usually heat required for initiation Initiator decomposition time should be considered - Amount of initiator, reaction temperature and initiator half-life (slow decomposition) Initiation Rate = Termination Rate - “steady state” kinetics apply Overall, [radical concentration] = low Since termination (disproportionation and coupling mechanism) is random, a broad MWD results. This polymer is dead (cannot initiate new monomer additions). Examples of Polymers Prepared by Radical Polymerization Monomer H H n Polymer CH2CH n Poly(styrene) H n H H CH2CH CN H H H n CH2CH H Poly(acrylonitrile) n CN C O MeO n O Poly(methylacrylate) OMe H H n H O Me CH2CH n O O C Me O Poly(vinylacetate) Advantages of Radical Polymerization 1. Wide variety of vinyl monomers can be polymerized (electron rich and deficient DBs) 2. Can be carried out in bulk and in a wide variety of solvents, which include water and organic solvents 3. No rigorous purification of reagents or drying of solvents required 4. Rapid formation of high molecular weight polymer after small conversions of monomer to polymer ( chain (addition) polymerization) 5. Living/controlled polymerizations enable easy formation of block copolymers and sophisticated architectures 75% of commercial polymers are made by radical polymerizations Some monomers can only be polymerized by radical means, e.g. acrylic acid (AA) H H H H C C AIBN C C H C O OH H n COOH Ion-exchange resins, smart polymers Radical Polarity Polar Effects are important in radical polymerizations, and can give alternating copolymers CN R R Ph Ph Ph CN R n Ph CN Ph CN Chain Reaction – initiation, propagation, termination Chain polymerization with termination e.g. conventional radical polymerization Life time of polymer radical chain is about 1 second Initiator added so to slowly decompose throughout polymerization time Typically, rate of initiation = rate of termination Therefore, [propagating radical] remains constant Steady State DP 100 50 conversion Chain polymerization without termination e.g. nitroxide-mediated radical polymerization (NMP) DP = DP “Living” [monomer] [Initiator] Initiator decomposes quickly, and polymer chains have long life times 100 50 conversion Nitroxide-mediated Controlled/living Radical Polymerization (NMP) ∆ P T + P T propagation T● = Nitroxide P● = Propagating radical T● is sterically congested Features: 1. Molecular weight increases linearly with conversion 2. Narrow molecular weight distributions obtained 3. Polymer chains contain living ends enabling chain extension or block copolymer synthesis Block copolymer synthesis AAAA n A T AAAA n A + T propagation Bn AAAA n A BBBB B n T Conventional Radical Polymerization Broad MWD Dead Polymer Controlled Radical Polymerization T T T T T Narrow MWD T Living Polymer Life time of radicals extended from 1 second to hours, as the radicals do not get involved in irreversible bimolecular termination reactions, since radicals are trapped by nitroxide reversibly Initiator must decompose quickly to insure narrow MWD Example of Block Copolymer Formation n-1 Ph Ph m O OMe D : n = 60 : m = 20 AIBN, heat SG1 Ph n n = 60 Ph First living poly(styrene) block heated in the presence of methyl acrylate to give diblock D P O O OEt n-1 Ph Please correct block copolymer structure in questions Reversible trapping added to propagation to prevent irreversible termination OEt N heat Ph n-1 Ph + N O OEt P OEt O SG1 propagation OMe m = 20 m O n-1 Ph Ph O N m O OMe D : n = 60 : m = 20 O P O O Me H C C H C Me n O CH2 C C AIBN MeO n O Poly(methyl methacrylate) OMe “perspex” MMA Nitroxides cannot control MMA Polymerizations H CH3 C + C CO2Me H PMMA N O OEt P OEt O SG1 H disproportionation C CH2 C H PMMA= + CO2Me OEt N P OEt OH O SG1-H McHale, Aldabbagh, Zetterlund, J. Polym. Sci. Part A: Polym. Chem. 2007, 45, 2194-2203 Alternating, Block and Graft Copolymers are made by radical copolymerization AIBN, heat excess small monomer macromonomer Graft Copolymer Graft Copolymer Formation Recent Example of a Graft Copolymer Synthesis Copolymerization + macromonomer monomer Poly(AA) Graft copolymer NIPAM H Br H H CH2 C CH2 C O C OH O C CH C n 2 C C OH H2C H + OCH2CH3 O Poly(acrylic acid) macromonomer CH H3C C O CH N H3C H N-Isopropylacrylamide NIPAM monomer (excess) Insoluble in water above the lower critical solution temperature (LCST) McHale, Aldabbagh, Carroll, Yamada, J. Polym. Sci. Part A: Polym. Chem. 2007, 45, 4394-4400 Copolymerization of Poly(AA) Macromonomer with NIPAM in ethanol at 60 ºC 0.6 W (log M ) 0.5 macromonomer 4% 0.4 0.3 63% 40% 19% 0.2 0.1 0.0 2 3 4 5 6 7 log M Shift to higher MW 8 Dual-Responsive “Smart” Graft Copolymer Copolymerization + macromonomer Poly(AA) Insoluble in water Monomer Graft copolymer NIPAM Soluble in NaOH (aq) Poly(NIPAM) in water (40ºC) Graft copolymer in NaOH solution (40ºC) Graft copolymer in NaOH (50ºC) McHale, Aldabbagh, Carroll, Yamada, J. Polym. Sci. Part A: Polym. Chem. 2007, 45, 4394-4400 Gibbons, Carroll, Aldabbagh, Yamada, J. Polym. Sci. Part A: Polym. Chem. 2006, 44, 6410-6418 Ziegler-Natta Chain (Addition) Polymerization H H C C H H TiCl4/AlEt3 1-4 atm, rt n Milder conditions than radical polymerization HDPE (high density poly(ethylene) is 3-10 times stronger than LDPE Less cross-linking, as terminal DBs less reactive than substituted DBs of radical polymerization Termination reaction H H Cl3Ti Few monomers polymerized by Z/N + Cl H Ti Cl Cl Ziegler-Natta Addition Polymerization Isotactic polymerization TiCl4 / AlR3 R 1-4 atm, rt n R Cl3Ti TiCl4 + AlR3 Cl3Ti AlR2 R + Cl scomplex AlR2 Cl R Cl3Ti Cl3Ti R Cl3Ti pcomplex Cl3Ti R Cl3Ti R Cl3Ti R Cl3Ti n R R Stereochemistry and Polymers M any useful pol ym e r s, suc h as pol y( st yr e ne ), poly(acrylonitrile) and poly(vinyl chloride) are atactic as normally prepared. Customized catalysts that effect stereoregular polymerization of poly(propylene) and some other monomers have been developed, and the improved properties associated with the increased crystallinity of these products has made this an important field of investigation. Amorphous polymer – melts to a hard rubbery, glassy state The properties of a given polymer will vary considerably with its tacticity. Atactic poly(propylene) is useless as a solid construction material, and is employed mainly as a component of adhesives or as a soft matrix for composite materials. In contrast, isotactic polypropylene is a high-melting solid (ca. 170 ºC) which can be molded or machined into structural components. Because poly(propylene) rope is so light, it is the only rope that floats. For this reason, it is very popular among ropes for pool makers and water sports. Also when wet it is flexible and does not shrink. Step-growth Polymerization Step-polymers are made by allowing difunctional monomers with c o m p l e m e n t a r y f u n c t i o n a l g r o u p s t o r e a c t w i th o n e an o t h e r Condensation between two molecules O HO O + MeO OMe terephthalic acid O O C C OCH2CH2O n OH ethylene glycol Poly(ethylene terephthalate) This is an example of a poly(ester) The reaction is a transesterification Using a condensation reaction PET Recyclable plastic bottles and textile fabrics Step-growth Polymerization These are poly(amides) – bristles of toothbrishes, stockings, rope, tires, carpet fibre Molten nylon spun into fibres - H2O 260-280 °C 250 psi First patented by Dupont MW = 10,000, m.pt. 250 °C, fibres stretched (to increase strength) to 4 times their length Self-Condensation or Ring-Opening Polymerization Also opened by cations & anions First patented by BASF High temp. to drive off water Nylon 6 is made by heating caprolactam to about 250 ºC with about 5-10% water Step-growth Polymerization 1. Polymers retain their functionality as end groups at the end of the polymerization 2. Only a single reaction is responsible for polymer formation 3. Molecular weight increases slowly even at high conversion. This is given by the Carothers equation, where conversion is (p) DP = 1 1-p At 98% conversion, the degree of polymerization is only 50% Larger chains react only at very high conversion 4. Exact stoichiometric balance and very pure monomers are required to achieve high molecular weights 5. Equilibrium reactions – necessary to remove by-product Step-growth Polymerization Step-addition – “no by-products” O C N N 180 °C O Insulation foam, HP adhesives, sealants, carpet underlay C O + Bayer-patented HO OH O N N H H O O n Poly(urethane) Lower Temp. than condensation reactions O CH2 O rt + CH2 6 6 n O bisdiene O benzoquinone Cyclic diene held cis is very reactive e.g. dicyclopentadiene Chain-growth condensation CH2N2 BF3 [ CH2 ]n + N2 Impurity found in diazomethane Time for litter to biodegrade Product Time to biodegrade Paper 2-5 months Wool socks 1 to 5 years Plastic coated paper milk cartons 5 years Plastic bags 10 to 20 years Nylon fabric 30 to 40 years Aluminum cans 80 to 100 years Plastic 6-pack holder rings 450 years Glass bottles 1 million years Plastic bottles Forever Plastic resin identification codes (1) Codes Descriptions Recycled products Polyethylene terephthalate (PET, PETE) is clear, tough, and has good gas and moisture barrier properties. Commonly used in soft drink bottles and many injection molded. Other applications include strapping and both food and non-food containers. Cleaned recycled PET flakes and pellets are in great demand for spinning fiber for carpet yarns, producing fiberfill and geo-textiles. Fiber, tote bags, clothing, film and sheet, food and beverage containers, carpet, strapping, fleece wear, luggage and bottles. High Density Polyethylene (HDPE) is used to make bottles for milk, juice, water and laundry products. Unpigmented bottles are translucent, have good barrier properties and stiffness, and are well suited to packaging products with a short shelf life such as milk. Because HDPE has good chemical resistance, it is used for packaging many household and industrial chemicals. Bottles; pipe, buckets, crates, flower pots, garden edging, film and sheet, recycling bins, benches, dog houses, plastic lumber, floor tiles, picnic tables, fencing. Polyvinyl Chloride or PVC has excellent chemical resistance, good weatherability, flow characteristics and stable electrical properties. The vinyl products can be broadly divided into rigid and flexible materials. Bottles and packaging sheet are major rigid markets, but it is also widely used as pipes and fittings, siding, carpet backing and windows. Flexible vinyl is used in wire and cable insulation, film and sheet, floor coverings synthetic leather products, coatings, blood bags, medical tubing and many others. Packaging, binders, decking, paneling, gutters, mud flaps, film and sheet, floor tiles and resilient flooring, cables, mats, cassette trays, electrical traffic cones, boxes, garden hose, mobile. Plastic resin identification codes (2) Codes Descriptions Recycled products Low Density Polyethylene (LDPE) used predominately in film applications due to its toughness, flexibility and relative transparency, making it popular for use in applications where heat sealing is necessary. LDPE is also used to manufacture some flexible lids and bottles and it is used in wire and cable applications. Shipping envelopes, garbage can liners, film and sheet, furniture, compost bins, paneling, trash cans, landscape timber, lumber Polypropylene (PP) has good chemical resistance, is strong, and has a high melting point making it good for hot-fill liquids. PP is found in flexible and rigid packaging to fibers and large molded parts for automotive and consumer products. Automobile battery cases, signal lights, battery cables, brooms, brushes, oil bins, funnels, bicycle racks, trays pallets, sheeting. Polystyrene (PS) is a versatile plastic that can be rigid or foamed. General purpose polystyrene is clear, hard and brittle. It has a relatively low melting point. Typical applications include protective packaging, containers, lids, cups, bottles and trays. Light switch plates, vents, thermal insulation, desk trays, rulers, license plate frames, foam packing, foam plates, utensils Other. Use of this code indicates that the package in question is made with a resin other than the six listed above, or is made of more than one resin listed above, and used in a multi-layer combination. Bottles, plastic lumber Recycling of plastic containers and wrapping Chemical Recycling by Eastman Kodak methanolysis O O C C OCH2CH2O CH3OH H+ n O HO O C MeO C + OMe PET These monomers are purified by distillation or recrystallization and used as feedstocks for further PET film manufacture. OH Representative Exam Questions 1. Using one appropriate monomer for each polymerization classification, discuss the mechanism and kinetics; (a) Step-growth, b) conventional (non-living) chain (addition), c) living chain (addition) polymerizations. In your answer give details of reaction conditions and reagents required. 2. (a) Discuss the stability of nitroxide radicals, and there use in living radical polymerizations. (b) Why is it not possible to control the radical polymerization of methyl methacrylate with nitroxides? 3. How would you prepare the following polymers? Give reaction conditions, reagents and detailed mechanisms for each polymerization. Name polymers A-D. n n n C B n-1 Ph Ph m O OMe D : n = 60 : m = 20 A 4. Draw structures of the polymers obtained from the following reactions; CO2Me + MeO2C O HO OH H+ KOH 5. Give one example of an isotatic polymer and block and alternating copolymer. Provide reactions (with conditions) and mechanisms for their synthesis.


![CHEE_392_-_Words_to_know-Processing[1] - P](http://s3.studylib.net/store/data/009652514_1-54ca9a81dd105bea22c19783eb204240-300x300.png)


