Document 9681882
advertisement

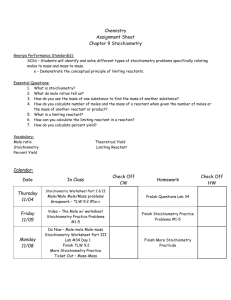
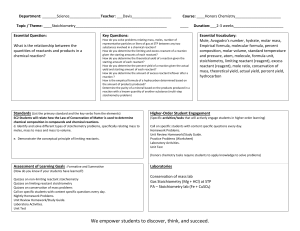
Study Buddy: Stoichiometry 1: Theoretical Terms Description Relative quantities of reactants, products and energy in a chemical reaction Diagram/Example Stoichiometry http://en.wikipedia.org/wiki/Stoichiometry Theoretical Yield Reaction: A + B C Theoretical: 5g + 5g 10g In the lab, 5g of each reactant were used and the reaction yielded 8g of C. (Actual yield) Calculated amount of reaction products Amount of product determined by laboratory experiment Ratio of amount of product from a reaction Percent Yield compared to the calculated yield Representations: Actual Yield 1. For the reaction a. Draw a particle diagram to represent the reaction % Yield: 8g/10g x 100% = 80% yield 2HCl + + Mg H2 + MgCl2 + b. Circle the ratios below are invalid for this reaction, and explain why they are not correct. 2 moles HCl 1 atom Mg 1 mole Mg 1 molecule H 1 mole MgCl 1 molecule H 1) 1 mole H 2) 1 mole MgCl 3) 1 mole H 4) 1 atom Mg 2 5) 1 mole HCl 2 6) 1 mole MgCl2 2 2 2 #2 is invalid because one atom of Mg would be proportional to one formula unit of MgCl 2, not a mole. The “scale” of the two units does not match. #3 is invalid because the subscript on Hydrogen was left off. #5 is invalid because the coefficient of HCl means each mole of MgCl2 would be proportional to 2 moles of HCl. #6 is invalid because the scale of the units does not match. 2. a. Rank the amounts of oxygen gas (O2) in increasing order: 1.2x1024 molecules, 32g, 1.5 moles least amount: __32g__, ___1.5 moles___, __1.2x1024 molecules__: most amount b. Rank the number of particles in 100g of: NaCl, CaCO3, FeCl3, MnO2 least amount: __FeCl3__, __CaCO3__, __MnO2__, __NaCl __: most amount 3. When potassium chlorate is heated it decomposes into potassium chloride and oxygen __2_KClO3(s) __2__KCl(s) + __3__O2(g) 2 moles KClO3= 245 g 2 moles KCl= 149 g Students performed this reaction in class and obtained 85g of KCl. a. How many grams of KCl should be produced from 165 grams of KClO3? Note: Students can use ratios or dimensional analysis to solve this type of problem. x g KCl 165 g KClO = 245 g KClO3 x = 100 g KCl 149 g KCl 3 b. Circle the theoretical yield. How would a student know this is the theoretical yield? The amount of expected yield from the reaction was calculated. c. Place a box around the actual yield. How would a student know this is the actual yield? The actual yield either comes from a lab or has to be given. d. What is the percent yield of potassium chloride for this reaction? Percent Yield=( actual yield theoretical yield )(100) Percent Yield=( 85 g KCl 100 g KCl )(100) = 85% yield KCl 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 1 Study Buddy: Stoichiometry 2: Applications Terms Description Substance added in excess and partially Excess Reactant consumed in a reaction Diagram/Example Excess = Substance totally consumed in a reaction and Limiting = Limiting Reactant limits the amount of products formed http://stoichiometricequiv.blogspot.com/2013/04/limitingreactantreagent.html Representations: 1. _1_ CH4(g) + _2_O2(g) _1_CO2(g) + _2_H2O(l) + 891 kJ 1 mol CH4 = 16 g 2 mol O2 = 64 g a. When using natural gas (mostly methane) to cook, which reactant is limiting and why? Normally methane is limiting because without oxygen humans cannot breathe and there are many more oxygen particles in a kitchen than those with which the methane can react. b. In a closed container, 100.g of methane and 100.g of oxygen gas are ignited by a spark. Which reactant is limiting and how do you know? Students could answer from calculations. However, from the balanced equation 64 g of O 2 is equivalent to 2 moles, which reacts with 1 mole of CH4 at 16 g. Based on the reaction ratio of 16g CH4: 64g O2, the reaction will run out of oxygen before methane. c. Draw particles to represent this reaction in the containers. Key: spark Reactants Before Reaction Carbon Hydrogen Oxygen Products After Reaction d. How many liters of carbon dioxide can be produced? From the balanced equation: 1 mole CO2 = 22.4 L CO2 x L CO2 100. g O = 64 g O 2 x = 35 L CO2 22.4 L CO 2 2 e. How many grams of the excess reactant will be used in the combustion? x g CH4 100. g O = 64 g O 2 x = 25 g CH4 16 g CH 4 2 2. What can cause the percent yield for a reaction to be less than 100%, other than human error? Especially with gaseous products, some of the products may not be collected from a reaction. 3. Draw particle diagrams to represent this reaction: _2_ KI (aq) + _1_ Pb(NO3)2 (aq) _1_ PbI2 (s) + _2_ KNO3 (aq) Key: K I Pb NO3 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL Precipitate of PbI2 copyright © 2015 CFISD 2 Units: Stoichiometry 1 & 2: Theoretical and Applications Planning Considerations Content: Unit side-by-side (show spiraling) Unit 1 (PROP) Mole concept Avogadro’s number Significant figures, scientific notation, units Particle diagrams for physical and chemical changes Unit 2 (STATES) Avogadro’s Law Unit 5 (SUBMIX) Molarity and calculations Unit 9 (BOND) Identify type(s) of bonding present Lewis valence electron dot structures Unit 10 (FORM) Molar mass of a compound Unit conversions for a compound Laws of definite and multiple proportions Unit 11 (ENRXN) Chemical and physical changes Writing chemical formulas Unit 12 (CLASSRXN) Conservation of mass through particle diagrams and balanced equations Identify reactions as precipitation or redox Precipitates and solubility rules Possible flow – conceptual development flow rationale Meaning of chemical equation coefficients Quantities in a chemical reaction Excess and limiting reactants Gas stoichiometry Reactions in aqueous solution This set of two units (STOICH1 and STOICH2) begins with student previous knowledge of properties associated with chemical changes and the importance of balanced chemical reactions. Particle diagrams and simulations are valuable for students to understand interactions between particles and visualize chemical processes at the particulate level. 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 3 This is also an opportunity to revisit student weaknesses in a new context for content from many previous units if they have not mastered the content yet. Students will revisit unit conversions for a single compound, then expand this to conversions between compounds in a balanced chemical equation. Before performing stoichiometry calculations, students should understand the relationships in shown in chemical equations at the particulate level. Students should be able to translate between particle diagrams, chemical equations and the physical reactions they represent and explain the information contained within each. As much as possible, students should perform reactions that are being discussed so they have a deeper understanding and can connect the reactions they are performing with the relevance chemical equations and particle diagrams. Once students understand conceptually, the additional layer of computation can be added on top using ratios and/or dimensional analysis. Students can approach stoichiometry calculations many different ways and should be free to use logical methods for arriving at answers. Dimensional analysis and ratios are two common methods used for calculations, but as long as students can justify and explain their process and it is scientifically valid then the method of arriving at an answer is not of key importance. For the calculations, use examples of reactions that go (or almost go) to completion; the TEKS do not include the teaching of chemical equilibrium or reversible reactions. Instruction and calculations should focus towards the understanding of the relationships between quantities (mole to mole ratio, mass to mole ratio, etc.) within a chemical reaction and use whole number multiples or quantities especially for L- level students when performing stoichiometry calculations. Stoichiometry is a supporting TEKS to the mole concept and law of conservation of mass readiness TEKS. Stoichiometry in this way is viewed as an extension of the law of conservation of mass and the mole concept to account for quantities in chemical reactions. STOICH2 begins with the concepts of limiting and excess reactants because now that students can convert between quantities of different chemicals in a reaction they can use this ability to determine which reactant will limit formation of products. This should be accomplished using particle diagrams and calculations to support each other. Once students have mastered determining the limiting and excess reactants, this layer can be added on top of stoichiometry calculations and students asked to determine from amounts of the reactants the amount of products that can be made, amount of products consumed, etc. The remainder of STOICH2 concerns the application of stoichiometry principles to reactions that produce gases and those that produce precipitates. As with the rest of the units, student calculations should be grounded in reactions they have physically performed and made measurements themselves. Use of gas law calculations should be to support students’ conceptual understanding of relationships being observed in reactions, especially if non-STP calculations need to be considered. Gas laws use and calculations should emphasize real-world relevance. Precipitation reactions provide another venue for students to apply their knowledge of limiting and excess reactants and an opportunity to measure the percent yield of a solid product from a laboratory experience. 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 4 Math/algebra Conversions between units for a single compound (g, mol, L, molecule, particles) Conversions between units for different compounds (dimensional analysis and/or ratios) Percent yield Limiting and excess reactants Molarity Misconceptions: Not distinguishing between grams and moles. Not distinguishing between coefficients and subscripts and their physical meanings. That all chemical reactions go to completion. Not distinguishing between experimental and theoretical yield. WEB resources: Classroom Resources (Can be used in the classroom for instruction) Limiting Reactant Animation (http://www.mhhe.com/physsci/chemistry/essentialchemistry/flash/limitr15.swf) PhET: Reactants Products and Leftovers (http://phet.colorado.edu/en/simulation/reactantsproducts-and-leftovers) Stoichiometry Applet (http://chemcollective.org/activities/simulations/stoich) Introduction to Limiting & Excess Reactant (http://islcs.ncsa.illinois.edu/media/com_resource/docs/Intro_to_Limiting_Reactants.pdf) Pearson Avagadro’s Cookies Untamed Science Video Student Resources (Students can use for review at home) Pearson Interpreting a Balanced Chemical Equation Interactivity Balanced Equation as a Recipe Student Tutorial Calculating the Mass of a Product Tutorial How Do You Fill an Air Bag? Pearson Flipped Video for Science Calculating Percent Yield Pearson Flipped Video for Science YouTube Bozeman Science: Stoichiometry (https://www.youtube.com/watch?v=LQq203gyftA) Bozeman Science: Limiting Reactant and Percent Yield (https://www.youtube.com/watch?v=LicEaaXhlEY) Crash Course Chemistry: Stoichiometry (https://www.youtube.com/watch?v=UL1jmJaUkaQ) Khan Academy Stoichiometry Practice Problems (https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/stoichiometryideal/e/ideal_stoichiometry) 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 5 POGIL: Flinn Scientific POGIL Activities for High School Chemistry (2012) Relative Mass and the Mole; Page 161 Mole Ratios; Page 169 Limiting and Excess Reactants; Page 175 PD Support: Share sessions, Team meetings Released STAAR/EOC Questions: 9, 12, 18, 27, 35 8th Grade STAAR Questions: none 3/17/15 SCIE_CHEM_STOICH1_MAT_STUDYBUDDYTE_AL copyright © 2015 CFISD 6