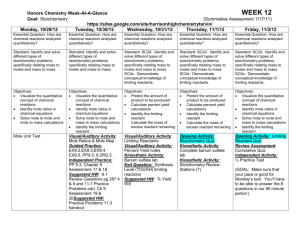
New Assign Sheet Stoichiometry
advertisement
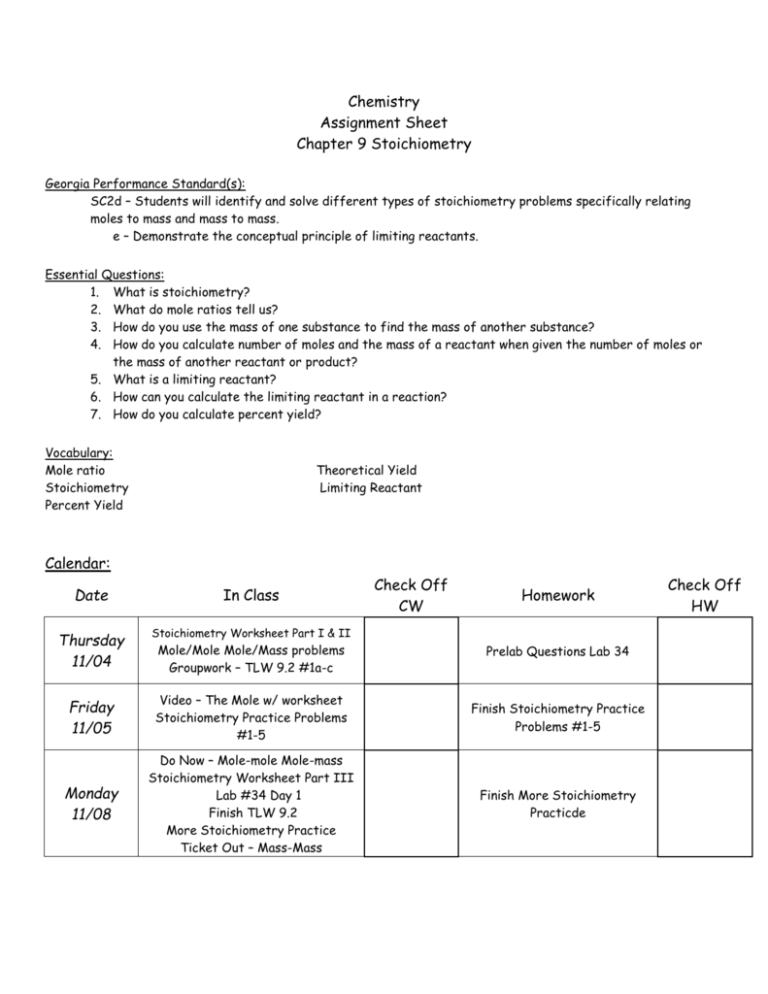
Chemistry Assignment Sheet Chapter 9 Stoichiometry Georgia Performance Standard(s): SC2d – Students will identify and solve different types of stoichiometry problems specifically relating moles to mass and mass to mass. e – Demonstrate the conceptual principle of limiting reactants. Essential Questions: 1. What is stoichiometry? 2. What do mole ratios tell us? 3. How do you use the mass of one substance to find the mass of another substance? 4. How do you calculate number of moles and the mass of a reactant when given the number of moles or the mass of another reactant or product? 5. What is a limiting reactant? 6. How can you calculate the limiting reactant in a reaction? 7. How do you calculate percent yield? Vocabulary: Mole ratio Stoichiometry Percent Yield Theoretical Yield Limiting Reactant Calendar: Check Off CW Date In Class Thursday 11/04 Stoichiometry Worksheet Part I & II Friday 11/05 Video – The Mole w/ worksheet Stoichiometry Practice Problems #1-5 Finish Stoichiometry Practice Problems #1-5 Monday 11/08 Do Now – Mole-mole Mole-mass Stoichiometry Worksheet Part III Lab #34 Day 1 Finish TLW 9.2 More Stoichiometry Practice Ticket Out – Mass-Mass Finish More Stoichiometry Practicde Mole/Mole Mole/Mass problems Groupwork – TLW 9.2 #1a-c Homework Prelab Questions Lab 34 Check Off HW Tuesday 11/09 Lab # 34 – Day 2 Procedure Review More Stoichiometry Practice Problems p. 312 #22 & 24 Wednesday 11/10 Cooties Activity Limiting Reactant Discussion/ppt Lab #34 – Day 3 Procedure Lab Calculations Ticket Out – Limiting Reactant Lab Report Thursday 11/11 Activity – S’mores Groupwork – Limiting Reactants/ Limiting Reactants & % Yield Ticket Out – Limiting Reactants U Can Pretend Itsa Test Friday 11/12 Review Problems Quiz - Stoichiometry Monday 11/15 Do Now – Limiting Reactants Review Stoichiometry problems Ticket Out – Percent Yield Tuesday 11/16 Unit Test – Chemical Quantities Read science article and answer comprehension questions p. 313 #34a, 35c, 36a, 46