Document
advertisement

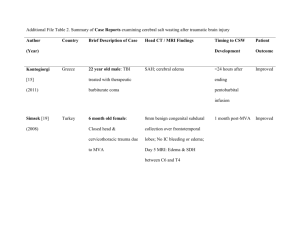
eEdE-35 WHAT RADIOLOGISTS SHOULD KNOW ABOUT BRAIN DEATH: RADIOLOGIC SIGNS OF A NONRADIOLOGIC DIAGNOSIS Joseph Gastala, MD; Aristides Capizzano, MD; Patricia Kirby, MD; Toshio Moritani, MD, PhD University of Iowa Hospitals and Clinics DISCLOSURES • The authors have no financial or nonfinancial relationships to disclose OUTLINE • Introduction • Definitions • Diagnostic criteria and ancillary testing • Pathophysiology and pathology • Imaging Findings • Summary INTRODUCTION • Concept of brain death was first described by Mollaret and Goulon in 1959 when they described “le coma depasse” of comatose patients supported by mechanical ventilators with absent electroencephalographic recordings, absent intracranial flow, or total brain necrosis at autopsy[1], basing death off neurologic criteria. • Early recognition of brain death is important to expedite organ transplantation, provide closure for loved ones, and prevent unnecessary negative medical interventions. • As will be discussed, brain death is a clinical diagnosis that is not always straightforward. Ancillary tests (EEG, angiography, transcranial Doppler, and scintigraphy) are included in the criteria as confirmed tests. However, radiologists usually encounter brain death or nearly brain death cases on other imaging modalities including CT, CT angiogram, CT perfusion, MRI, MR angiogram and diffusion weighted imaging, which often demonstrate characteristic findings. • This educational exhibit will illustrate pathophysiology and pathology and demonstrate the utility of multimodal imaging findings. Radiologists should understand brain death and their role in recognizing, reaching, and expediting the diagnosis. DEFINITIONS • The definition of “brain death” according to the Uniform Determination of Death ACT[2] • “Irreversible cessation of functions of the entire brain, including the brain stem” • As a purely biologic definition, “death” is the irreversible cessation of critical functions in an organism as a whole[3]. • The equivalence of death with brain death is not universally accepted, but it has been accepted throughout most of the Western world[4, 5, 6] PREREQUISITES FOR THE DIAGNOSIS • The diagnosis of brain death requires consideration of the clinical setting and that the cause of brain death be known; this is often elucidated by CT scan[7]. In patients with coma of undetermined origin, the diagnosis of brain death can be difficult and complex. • The irreversibility of the patient’s clinical state needs to be established[8, 9] • Mimics and reversible causes include[8]: • Locked in syndrome • Hypothermia • Drug intoxication • Severe electrolyte, acid-base, and endocrine disturbances Prerequisites Neurologic Exam Ancillary Testing DIAGNOSTIC CRITERIA • The diagnosis of brain death is made on clinical grounds and neurologic exam. In the United States, clinical criteria have been set forth by the American Academy of Neurology [7, 8] • Documentation of coma • Absence of motor responses to painful stimulus • Supraorbital nerve pressure, temporomandibular joint pressure, sternal rub, or nail-bed pressure • Absence of brain-stem reflexes • Absence of pupillary responses, corneal reflexes, caloric responses, gag reflexes, coughing in response to tracheal suctioning, sucking and rooting reflexes • Apnea • Absence of respiratory drive Prerequisites Neurologic Exam Ancillary Testing WHEN THE DIAGNOSIS IS UNCERTAIN • Sometimes the clinical criteria cannot be applied due to uncertainty about the reliability of the neuroogic examination, or when the apea test cannot be performed. These situations include[9,11]: • Situations in which cranial nerves may not be adequately examined, e.g. severe facial trauma, preexisting pupillary abnormalities • Cases in which neuromuscular paralysis is present or when profound metabolic and endocrine disturbances are not readily reversed • Patients with severe pulmonary disease resulting in carbon dioxide retention rendering the apnea test nondiagnostic • In these cases, ancillary testing should be considered Prerequisites Neurologic Exam Ancillary Testing ANCILLARY TESTS FOR BRAIN DEATH • There are two general categories of confirmatory tests for brain death[10]. There are four ancillary tests that have been approved by the American Academy of Neurology: • Confirmation of loss of electrical activity • Electroencephalography (EEG) • Demonstration of loss of cerebral blood flow • Cerebral angiography • Transcranial Doppler (TCD) ultrasonagraphy • Cerebral scintigraphy • Other studies have not yet been approved by the American Academy of Neurology • However, findings on MRI/MRA and CTA have been shown to correlate with brain death, and these findings must be recognized by the radiologist Prerequisites Neurologic Exam Ancillary Testing ELECTROENCEPHALOGRAPHY • Widely available and used in the determination of brain death in many countries • Electrical activity will be absent above 2 uV at a sensitivity of 2 uV/mm with a filter setting at 0.1 or 0.3 s and 70 Hz • Artifacts can be common if high gain amplification is used CEREBRAL ANGIOGRAPHY • Generally accepted as the gold standard among techniques that measure intracranial flow[40] • No cerebral filling should be demonstrated above the level of carotid or vertebral artery • Invasive, requires specialized skill, not always readily available TC-99M HMPAO SCINTIGRAPHY • Noninvasive, excellent correlation with cerebral angiogram • Multiple flow patterns have been described[12] • No intracranial flow provides straightforward confirmation of brain death; flow within cerebrum and cerebellum rules out the diagnosis • Preservation of cerebellar flow with absence of cerebral flow is not diagnostic, but patients will usually end up progressing to brain death • Lack of cerebellar flow with preservation of cerebral flow is an indeterminate finding[13] • Hot nose sign due to obstruction of internal carotid artery flow and subsequent increased external carotid flow into collateral and extracranial circulation through center of the face [14] • Disadvantages are that it is not widely available on an urgent basis Tc-99m HMPAO scintigraphy in a patient with clinically diagnosed brain death demonstrates no intracranial vascular flow. TRANSCRANIAL DOPPLER • Noninvasive and inexpensive, with great portability • Cerebral circulatory arrest is indicated by flow patterns without forward flow progress, progressing from 1) decrease in diastolic flow, 2) disappearance of diastolic flow, 3) oscillating pattern with retrograde flow in diastole, 4) short systolic spikes, and finally 5) absence of Doppler signal[15] • Has been shown to correlate with four vessel angiography in intracranial circulatory arrest[15]. Cerebral circulatory arrest in the basilar and both middle cerebral arteries correctly predicted fatal brain damage in all patients in one study[16] • Disadvantage of TCD is that it is highly operator dependent TCD patterns of cerebral circulatory arrest, taken from the right middle cerebral artery. A) Low diastolic velocity, B) systolic peaks with zero diastolic velocity, C) oscillating pattern with negative flow in diastole, D) short systolic spikes. Reprinted with permission from Hadani et al. [17] ETIOLOGY • Brain death is preceded by a massive, irreversible brain injury[18] • Most common causes of brain death in adults are traumatic brain injury and subarachnoid hemorrhage • In the ICU, brain death is most commonly seen in large ischemic strokes or hypoxic-ischemic encephalopathy • This injury leads to edema and massive destruction of tissue despite continued advanced life support GROSS PATHOLOGY • Brain herniation occurs due to elevated intracranial pressure • Tonsillar herniation • Uncal herniation • Transtentorial herniation • Transtentorial herniation leads to compression of the brainstem with stretching and laceration of pontine perforating branches of the basilar artery or thrombosis and venous infarction. These lead to duret hemorrhages • Brain will increasingly take on a dusky, congested, and discolored appearance once intracranial blood flow has arrested[40] Gross pathology specimens of tonsillar transtentorial herniation Gross pathology demonstrating uncal herniation Duret hemorrhages MICROPATHOLOGY • Diffuse cytotoxic edema occurs throughout the gray and white matter, with intracellular edema occurring within the astrocytes in gray matter and within the oligodendroglial cell bodies, astrocytes, myelin sheaths, and axons in white matter[27] • Interstitial edema occurs in periventricular tissues presumably from CSF reabsorption and increased transependymal flow • Autolysis is a phenomenon that occurs with anoxia related to release of intracellular compounds, which can be a result of delayed fixation[40], and can occur along with changes from brain death A B C Micropathology slides show A) vacuolation of white matter and B) decreased myelin staining (LFB stain). Compare this with C) normal controls (LFB stain). PATHOPHYSIOLOGY OF BRAIN DEATH Edema Increased ICP Cerebral circulatory arrest Electrocerebral silence MECHANISM OF BRAIN EDEMA Inciting process: Trauma, hypoxia, hemorrhage, tumor, toxicity • • • • Disruption of bloodbrain barrier Vasogenic edema Biomolecular mediators • of injury Cytotoxic edema An inciting process generates cerebral edema or mass effect. Cerebral blood flow is dictated by local autoregulatory mechanisms, related to regional concentrations of CO2[19]; the inciting process disturbs these mechanisms Disturbances in blood flow, oxygen, or glucose supply causes loss in neural and blood-brain barrier function[20] Additionally, occlusion of vessels induces a change in blood gas values and pH with associated acidosis and increase in lactate[20] These changes lead to edema. Classically, two types of edema are described • Vasogenic edema • Extracellular edema caused by influx of water when blood-brain barrier is disrupted • Decreased cerebral perfusion causes alteration in blood gases with increased pCO2 and causing secondary arterial vasodilation, contributing to extracellular water and edema [21] • Cytotoxic edema • Intracellular edema of glial cells • Metabolically mediated[20] • The combination of vasogenic and cytotoxic edema increases brain volume[21], and have the potential to increase intracranial pressure. Edema is greatest by 24 to 72 hours after the event [19] Brain Edema • CYTOTOXIC EDEMA AND CELL DEATH Inciting event The molecular cascade leading to cytotoxic edema and subsequent changes leading to cell death is complex, and the following is an oversimplification. Decreased local or global cerebral perfusion, traumatic injury • Cytotoxic Edema • Normally, Na/K ATPase pump creates an intracellular/extracellular sodium and potassium gradient • Decreased global or local perfusion causes decreased clearance of lactate and increased acidosis. • Failure of ATP production occurs due to ischemia, in addition to membrane depolarization and acidosis. This causes subsequent failure of the Na/K pump • There is subsequent shift of K+ into extracellular space, and Na+ ions and subsequently water into intracellular space[22] • Glutamate • Traumatic brain injury is associated with large quantities of extracellular glutamate due to failure of presynaptic ion pumps and calcium-mediated exocytosis[21] • Ischemic neuronal injury also induces release of large quantities of glutamate into extracellular space of the brain due to failure of the glutamate transporter, which normally maintains the normal glutamate gradient within neurons [22, 24] Glutamate Na/K ATPase Na+ Water Cytotoxic edema Failure of of electron transport chain Mitrochondria CYTOTOXIC EDEMA AND CELL DEATH Inciting event The molecular cascade leading to cytotoxic edema and subsequent changes leading to cell death is complex, and the following is an oversimplification. Decreased local or global cerebral perfusion, traumatic injury • Ca2+ Glutamate Calcium • Glutamate opens channels for calcium entry with binding to ionotropic receptors [25, 26] • Intracellular calcium subsequently enters the mitochondria through a calcium uniporter, and mitrochondrial calcium increases Receptor activation Na/K ATPase Oxygen radicals Increased Ca2+ Na+ Water Cytotoxic Apoptotic edema cascade Failure of of electron transport chain Mitrochondria • Mitochondria and cell death • Increased intramitochondrial calcium causes generation of reactive oxygen species[21] which further damages lipids, proteins, and DNA of the cell • High intramitochondrial concentration of Ca2+ causes release of cytochrome C with further alteration of electron transport and further decreases in energy [22, 25] • Cytochrome C and other proteins activate the apoptotic cascade leading to cell death PATHOPHYSIOLOGY OF BRAIN DEATH Edema Increased ICP Cerebral circulatory arrest Electrocerebral silence INCREASED INTRACRANIAL PRESSURE • Edema and mass effect, when extensive, cause associated rises in intracranial pressure • Cerebral perfusion pressure (CPP) is the driving arterial pressure gradient across cerebral vasculature, and is related to mean arterial pressure (MAP) and ICP[21] • CPP=MAP-ICP • Increasing intracranial pressure decreases CPP Edema and mass effect Decreased CPP Increased intracranial pressure Arteriole vasodilation Increased CBV Cerebral circulatory arrest • Autoregulation is the process of maintaining cerebral blood flow over varying cerebral perfusion pressures. Arteriole vasodilation is the response to maintain cerebral blood flow over decreased cerebral perfusion pressures. • This vasodilation leads to an increase in cerebral blood volume • A vicious cycle may ensue in which increases in CBV cause further increases in ICP, and the cycle begins again by decreasing CPP INTRACRANIAL PRESSURE AND INTRACRANIAL VOLUME REGULATION • Total intracranial volume = Blood + CSF + Brain tissue + Water[20] • The rigid cavity of the skull leaves very limited ability to compensate for swelling, edema, or mass effect. [21] • Compensatory mechanisms for increased brain volume include decreasing CSF (decreased production, increased absorption, shunting to spinal subarachnoid space) or shunting venous blood [21] • The main compensatory process for restoring equilibrium is CSF reabsorption. When most of the CSF has been reabsorbed, the brain will occupy the areas previously occupied by CSF, leading to herniation[20] • Herniation leads to loss of consciousness and eventually respiratory failure, which further exacerbates ischemia and contributes to further increases in intracranial pressure. • Even in processes of global ischemia and multifocal processes, there are differences in perfusion from one region of the brain to another[19], and not all areas of the brain are affected equally and uniformly. PATHOPHYSIOLOGY OF BRAIN DEATH Edema Increased ICP Cerebral circulatory arrest Electrocerebral silence CEREBRAL CIRCULATORY ARREST • Eventually venous congestion results, causing further increases in ICP. • As edema develops, a threshold is reached in which ICP rises exponentially to small changes in edema [29] • When intracranial pressure exceeds diastolic pressure, there is loss of perfusion[29, 30] ELECTROCEREBRAL SILENCE • Cerebral circulatory arrest leads ultimately to electrocerebral silence • Brain death is always accompanied by electrocerebral silence, although not all cases of electrocerebral silence are brain death[31] RADIOLOGIC IMAGING FINDINGS • Most of the imaging findings are related to increased intracranial pressure and associated cerebral circulatory arrest • It is important for the radiologist to recognize signs of brain death to suggest and expedite the diagnosis of brain death • MRI/MRA, CTA and CT perfusion studies have been shown to correlate well with brain death CT/CTA/CT PERFUSION • Noninvasive, widely available, great rapidity, and does not require a high level of expertise to perform • Accuracy first demonstrated by Dupas et al.[36], who first introduced a 2-phase protocol (arterial and mixed arterial and venous phase), with a 7 point scale. This was subsequently was accepted as one of the ancillary tests for confirmation of brain death in France. Additionally Austria, Switzerland, and Canada have adopted its use in confirmation of brain death. • Frampas et al introduced an abbreviated 4 point scale, concluding lack of opacification of MCAs and internal cerebral veins in CTA is efficient and reliable in brain death diagnosis [37] • Residual brain perfusion can occur with decompressive craniectomy and skull defects, leading to false positive results, and Welschehold et al. have proposed using lack of opacification of the ICV as criteria, showing high sensitivity and specificity[38] • Shankar et al. demonstrated lack of cerebral blood flow and cerebral blood volume in the brain stem with CT perfusion is very sensitive for brain death[39] • Disadvantages of the use of CTA are risk of renal damage for patients in consideration for organ transplant 43 YEAR OLD MALE WITH AORTIC DISSECTION, POSTOPERATIVE COURSE COMPLICATED BY ISCHEMIC BOWEL A, B) Axial slices of a CT angiogram demonstrate lack of opacification of intracranial circulation, including internal cerebral veins and midline arteries. There is opacification of the superficial temporal artery and branches (green arrows) A B C, D) Normal Axial CT angiogram of a normal patient for comparison demonstrating opacification of all intracranial arteries and internal cerebral veins (blue arrows) C D 57 YEAR OLD FEMALE, INITIALLY PRESENTING WITH HEADACHES, BECAME UNRESPONSIVE C B A D A) Axial noncontrast CT demonstrates subdural hematoma (blue arrow) and midline shift (blue arrow). B, C) Coronal and D) 3D CTA reconstructions demonstrate lack of opacification of intracranial circulation 42 YEAR OLD FEMALE WITH WITNESSED FALL WHILE RUNNING, FOUND TO HAVE DIFFUSE SAH A, B, C) Axial 3D CTA reconstructions demonstrate minimal delayed opacification of intracranial circulation more likely “nearly” brain dearth. C B A D) Cerebral Blood Volume and E) Cerebral Blood Flow CT perfusion images demonstrate matched decreased CBV and CBF within the supratentorium and posterior fossa D E MRI/MRA • A number of signs have been associated with the diagnosis of brain death on MRI and MRA [32, 33, 34, 35] • Massive brain edema leads to transtentorial and foramen magnum herniation • The increase in intracranial pressure and cerebral circulatory arrest leads to absence of intracranial vascular flow • There is poor gray matter/white matter differentiation with the brain edema on conventional MRI • Diffuse diffusion restriction results from diffuse cytotoxic edema, there is decreased ADC values more prominent in white matter compared to gray matter, related to differences in water accumulation in different cytoarchitectures [27] • Transcortical and transcerebral vein signs can be seen, which has also been described with acute stroke[35] • Prominent nasal and scalp enhancement • MRA can demonstrate non-visualization of intracranial vasculatures likely compatible with conventional angiogram. • Disadvantages of MRI /MRA include difficulty to obtain on ventilated patients and length of time for scanning 48 YO FEMALE WITH PERFORATED COLON SECONDARY TO BOWEL IMPACTION S/P COLOSTOMY, ADMITTED WITH AMS AND FOUND TO BE SEPTIC A B C D A) Sagittal T1W image shows foramen magnum and transtentorial herniation (green arrows), with massive brain edema. There is loss of gray white differentiation (orange arrow). B) Axial T2w shows loss of internal carotid artery intracranial flow voids (yellow arrow). C, D) Axial DWI and ADC map show diffusion restriction and low ADC values more prominent in the white matter more than cortex. E, F) Axial GRE and SWI show transcortical (thin blue) and transcerebral (fat blue arrows) vein signs[35]. E F 32 YEAR OLD MAN PRESENTING WITH HEADACHE, LUMBAR PUNCTURE WITH CSF CONSISTENT WITH VIRAL INFECTION A A) Sagittal T1W image shows tonsillar herniation (blue arrow) and loss of cortical sulci (orange arrow). B) Intracranial MRA demonstrates loss of vascular flow within the supraclinoid internal carotid arteries. B C, D) Axial DWI and ADC map demonstrate diffuse diffusion restriction with decreased ADC values. C D Vacuolation and decreased myelin staining in white matter SUMMARY • Early recognition of brain death is important to expedite organ transplantation, provide closure for loved ones, and prevent unnecessary negative medical interventions. • Brain death is a clinical diagnosis which can be a difficult diagnosis in the presence of confounding factors. Ancillary tests (EEG, Angiogram , TCD, Scintigraphy) are used to aid in confirmation of the diagnosis • We illustrated pathophysiology and pathology and demonstrated the utility of multimodal imaging findings including CT, CT angiogram, CT perfusion, MRI, MR angiogram and diffusion weighted imaging. • Radiologist can play a significant role in recognizing, reaching, and expediting the diagnosis. BIBLIOGRAPHY 1. Mollaret P, Goulon M. Le coma depasse. Rev Neurol 1959;101:3-15. 2. Guidelines for the determination of death: report of the medical consultants on the diagnosis of death to the President’s com mission for the study of ethical problems in medicine and biochemical and behavioral research. JAMA 1981; 246: 2184-2186 3. Bernat JL. A defense of the whole-brain concept of death. Hastings Cent Rep 1998:28;14-23. 4. Canadian Neurocritical Care Group. Guidelines for the diagnosis of brain death . Can J Neurol Sci 2000;26:64-66 5. Haupt WF, Rudolf J. European brain death codes: a comparison of national guidelines. J Neurol 1999;246:432-7. 6. Goldbeck-Wood S. Germany passes new transplant law. BMJ 1997;315:11. 7. The Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters for determining brain death in a dults (summary statement). Neurology 1995;45:1012-4. 8. Wijdicks, EF. The diagnosis of brain death. NEJM 2001;344(16):1215-21. 9. Wijdicks, EF et al. Evidence-based guideline update: Determining brain death in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010;74:19111918 10. Van der Lugt, A. Imaging tests in determination of brain death. Neuroradiology 2010;52:945-947 11. Young, BG, Lee, D. A critique of ancillary tests for brain death. Neurocrit Care 2004;1:499-508 12. Conrad GR, Sinha P. Scintigraphy as a confirmatory test of brain death. Semin Nucl Med 2003;33(4):312-323. 13. Mrhac L, Zakko S, Parikh Y. Brain death: The evaluation of semi-quantitative parameters and other signs in HMPAO scintigraphy. Nucl Med Commun 1995;16:1016. 14. Mishkin FS, Dyken ML. Increased early radionuclide activity in the nasopharyngeal area in patients with internal carotid artery obstruction: “h ot nose.” Radiology 1970;96:77-80. 15. Hassler W, Steinmetz H, Pirschel. Transcranial Doppler study of intracranial circulatory arrest. J Neurosurg 1989:71(2):195-201. 16. Monteiro LM, Bollen CW, van Huffelen AC, Ackerstaff RG, Jansen NJG, van Vught AJ. Transcranial Doppler ultrasongraphy to confirm brain death: a meta-analysis. Intesnive Care Med 2006;32:19371944. 17. Hadani M, Bruk B, Ram Z, Knoller N, Spiegelmann R, Segal E. Application of transcranial Doppler ultrasonography for the diagnosis of brain death. Intensive Care Med 1999;25:822-828. 18. Wijdicks, EF. Determining brain death in adults. Neurology 1995;45:1003-1011 19. Sioutos PJ, Orozco JA, Carter LP. Regional cerebral blood flow techniques. In: Narayan RK, Wilberger Jr JE, PovlishockJT, eds. Neurotrauma. New York: McGraw-Hill, 1996:503-517. 20. Leestma, JE. Neuropathology of brain death. In: Wijdicks EF, editor. Brain Death. Philadelphia: Lippincott Williams & Wilkins, 2001:45-60. BIBLIOGRAPHY 21. Greve MW, Zink BJ. Pathophysiology of traumatic brain injury. Mount Sinai Journal of Medicine 2009. 76;97-104. 22. Oechmichen M, and Meissner C. Cerebral hypoxia and ischemia: The forensic point of view: A review. J Forensic Sci 2006;51:880-7. 23. Kurtek, RW, Lai KK, Tauxe WN, Eidelman B, Fung JJ. Tc-99m Hexamethylpropylene amine oxime scintigraphy in the diagnosis of brain death and its implications for the harvesting of organs used for transplantation. Clinical Nuclear Medicine 2000;25(1):7-10. 24. Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. J Cereb Blood Flow Metab 1985;5:413-9 25. Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma 2000;17:843-55. 26. Rosenberg G. Ischemic brain edema. Progress in Cardiovascular Diseases 1999;3(42):209-216. 27. Selcuk H, Albayram S, Tureci E, Hasiloglu ZI, Kizilkilic O, Cagil E, Kocer N, Islak C. Diffusion-weighted imaging findings in brain death. Neuroradiology 2012;54:547-554. 28. Walker EA. Pathology of brain death. Annals New York Academy of Sciences 1978;315:272-280. 29. Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurg Focus 2007:22;1-10 30. Wijdicks, EF, Pfeifer ,EA. Neuropathology of brain death in the modern transplant era. Neurology 2008;70:1234-7. 31. Bennett DR. The EEG in determination of brain death. Annals New York Academy of Sciences 1978;315:110-120. 32. Jones KJ, Barnes PD. MR diagnosis of brain death. AJNR 1992;13:65-66. 33. Orrison WW, Champlin AM, Kesterson OL, Hartshorne MF, King JN. MR ‘hot nose sign’ and ‘intravascular enhancement sign’ in brain death. AJNR 1994;15:913-916 34. Karantanas AH, Hadjigeorgiou GM, Paterakis K, Sfiras D, Kommos A. Contribution of MRI and MR angiography in early diagnosis of brain death. Eur Radiol 2002;12:2710-2716. 35. Chul-ho, S et al. Imaging findings of brain death on 3-Tesla MRI. KJR 2012;13(5):541-9. 36. Dupas B, Gayet-Delacroix M, Villers D, Antonioli D, Veccherini M, Soulillou J. Diagnosis of brain death using two-phase spiral CT. AJNR 1998;19:641-647. 37. Frampas E, Videcoq M, de Kerviler E, Ricolfi F, Kuoch V, Mourey F, Tenaillon A, Dupas B. CT angiography for brain death diagnosis. AJNR 2009;30:1566-1570. 38. Welschehold S et al. Computed tomographic angiography as a useful adjunct in the diagnosis of brain death . J Trauma Acute Care Surg 2013;74:1279-1285. 39. Shankar JJs, Vandorpe R. CT perfusion for confirmation of brain death. AJNR 2013;34:1175-1179 40. Bradac GB, Simon RS. Angiography of brain death. Neuroradiology 1973;5:13-19.