Second Semester Review

Second Semester Review
Biotic factors
• Factors that are living or come from living organisms.
• Examples:
• Tree
• Wooden toothpick
• Paper
Abiotic factors
• Factors that are non-living, do not come from living organisms.
• Examples:
• Soil (rocks too)
• Air (climate, weather)
• Water (solid, liquid or gaseous water)
• Sunlight (light, temperature)
Levels of organization
• Smallest to largestorganism, population, community, ecosystem, biome, biosphere.
• Organism-Single member of species
• Population- Members of a species in a region
• Community- Different species interacting with one another in a specific area.
• Ecosystem- Biotic and abiotic factors in a given area.
• Biome-interacting ecosystems in a region.
• Biosphere- the part of Earth that supports life.
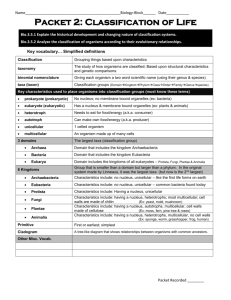
Classification
• Heterotrophic/ Autotrophic (TEKS 6.12D)*
• Heterotrophic/ Autotrophic
• Heterotrophic- gets nutrition from an outside source
• Examples are anything that eats: consumers, animals, bacteria, fungi
• Autotrophic- self feeder; makes own food through photosynthesis or chemosynthesis
• Examples are producers; plants and some acheaebacteria
Classification
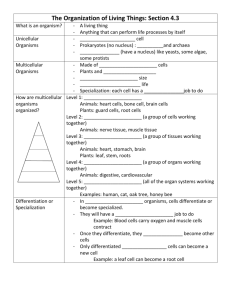
• Unicellular/Multi-cellular (TEKS 6.12D)*
• Organisms are composed of one or more cells: Unicellular/ Multicellular
• Unicellular- one celled organisms; the individual organism is made up of only one cell like bacteria.
• Multi-cellular- many celled organisms; the organism is made up of many cells working together like humans.
• Cells tissues organs systems organism
Classification
•
• Mode of Reproduction (TEKS 6.12D)*
• Sexual reproduction
• Sex cells are created through meiosis
• makes 4 daughter cells with half the genetic information
• Needs the genetic information of two parents to create a unique organism allowing for more genetic variation.
• Asexual reproduction
• Requires the genetic material of only one parent
• Offspring is genetically identical to the parent
• Types of asexual reproduction:
• Budding – organism grows out of the adult
• Binary fission- like mitosis, occurs in bacteria
• Parthenogenesis- whip tail lizards in New Mexico are all females and lay eggs that hatch to be clones of the mother.
• Regeneration- The regrowth of a missing limb, like lizard tails and starfish.
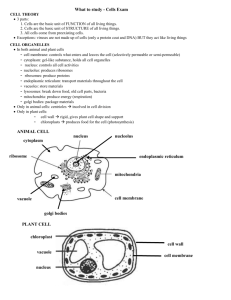
Life – the basic unit of all life is the cell. All organisms are made up of one or more cells.
Prokaryotic cells Eukaryotic cells
1. Do not have a nucleus, have a 1. Have a nucleus primitive loose genetic material.
2. Have membrane bound cell parts.
2. Do not have membranes around cell parts.
3. Protista, Fungi, Plantae, Animalia are kingdoms composed of eukaryotic organisms.
3. Always Unicellular (single celled) organisms like
Eubacteria and Archaebacteria.
4. Can be unicellular or multicellular
5. Can reproduce sexually or asexually
4. Reproduce asexually
The three DOMAINS
Archaea Bacteria
• Kingdom Archaebacteria • Kingdom Eubacteria Eukarya
•
• Single celled
Prokaryotic(no nucleus) •
• Single celled
Prokaryotic(no nucleus)
• Very primitive
• Asexual reproduction through binary fission.
• Live in extreme environments like: hot springs, around deep sea volcanic vents, mineral rich water. Cannot live in oxygen.
• Asexual reproduction through binary fission.
• Live in the soil, water, air and inside living organisms.
• Kingdoms:
• Plant
• Animal
• Protista
• Fungi
Have nucleus in cell
Can be multicellular or unicellular.
Are Eukaryotic (cells have nucleus)
Kingdom Plantae
• Are eukaryotic (cells have a nucleus)
• Majority are multicellular, reproduce sexually (pollination)or asexually
(budding).
• Autotrophic (makes own food)
• Have chloroplast
Kingdom Animalia
• Are eukaryotic (cells have a nucleus)
• Heterotrophic (get nutrition from outside sources)
• Multicellular
• Majority reproduce sexually (50% of the DNA comes form one parent and 50% from the other parent.
Kingdom Fungi
• Eukaryotic (nucleus)
• Heterotrophic
• Get their nutrients from decaying matter
• Unicellular or multicellular
• Can reproduce sexually or asexually
Kingdom Protista
• Eukaryotic
• Majority are Unicellular, very few are multicellular
• Have characteristics of plants and animals
• Autotrophs and/or heterotrophs
• Asexual reproduction through
• Budding, regeneration
• some reproduce sexually
Atoms- basic building block of all matter, the smallest whole unit of matter.
Charge
Atomic number is the number of
protons in the nucleus of an atom.
In a normal atom, the atomic number
= the number of protons = the number of electrons.
Protons and neutrons are found in the nucleus of an atom.
Electrons are found orbiting the nucleus in energy shells.
A chart that organizes the elements according to size
(mass)and physical and chemical properties.
Periodic table
Properties of Metalloids
• Have characteristics of both metals and non metals
• semiconductors
Properties of Metals
• Malleable
• Ductile
• Shiny
• Good conductors of heat and electricity
Properties of non-
Metals
• Brittle solids
• Dull
• Mostly gases
• Poor conductors of heat and electricity
• Good insulators
Elements comprise Earth (TEKS 6.5B)
How many elements are in the following compounds?
• Hint- count the capital letters. Each symbol should have only one capital letter. The symbol is the element’s abbreviation and it’s used in chemical formulas. Elements have only one to three letters in their symbols but only one is a capital letter.
• H
2
PO
4
• CO
2
• H
2
O
• C
6
H
11
• NH
3
O
2
How many elements in the following chemical reaction?
CO
2
+ H
2
O C
6
H
11
O
2
+ O
2
Matter
• Elements • Compounds
• A substance consisting of atoms which all have the same number of protons - i.e. the same atomic
• Substances made of two or more different kinds of atoms chemically bound together.
number. Elements are chemically the simplest form.
• Examples: water, salt, sugar,
• Examples: gold, copper, carbon, carbon dioxide, baking soda aluminum, oxygen, hydrogen, etc.
• The symbol is one letter as in H, O, C, or
• The formula has letters and numbers: H
2
O, NaCl, CO
2 two letters as in He, Ca, Ne, Cl
Physical properties of matter
1. Can be identified just by observing matter. You do not have to change it in any way.
2. Can be things you identify with your senses, such as, sight, touch, taste, smell, or hearing.
3. Include things such as color, texture, shape, mass, odor, state of matter, freezing point, boiling point, density, etc.
Review Physical Properties (TEKS 5.5A)
• Properties that can be measured or seen through direct observation, include;
• Size
• Color
• Luster
• Density
• Mass
• Volume
• Length
• Texture
• State (S,L,G)
• Malleability
• Magnetism
• Specific gravity
• Conductivity
• Temperature
• Hardness
• Cleavage
• Fracture
Examples of physical properties
• The way the squishy stuff stretches out
• The way a rubber ball bounces
• The way ice cream melts
• The sweet taste of candy
• The heaviness of a rock
• The rough texture of sandpaper
• The height of a building
• The hardness of a mineral, its streak,
• Magnetism
•
Density
The amount of matter in a given volume
• D=mass/volume
High density Medium density Low density
B
C
Liquid Mass
(g)
A 20.0
5.0
30.0
Volume
(mL)
10.0
Density
(g/mL)
25.0
30.0
After calculating the density of the three liquids, can you predict the order the liquids will be in if you used them to create a density tower?
Which liquid would be on the top? Explain.
Which would be on the bottom? Explain.
Physical states of matter
Solids Liquids
• Have a definite shape
• Have definite volume
• The atoms are really close together.
• Have indefinite shape
• Have definite volume
• The atoms are loosely held together.
Gases
• Have indefinite shape
• Have indefinite volume
• The atoms are far apart from each other.
Physical change
• Phase Change – aka change in state of matter – a PHYSICAL CHANGE
• Melting point – solid state to liquid state (heat is added)
• Boiling point – liquid state to gas state (heat is added)
• Freezing point – liquid state to solid state (heat is removed)
• Condensation point – gas state to liquid state (heat is removed
Chemical properties of matter
1. You have to change matter in some way to identify a chemical property.
2. You cannot tell about chemical properties by just using your senses.
3. Includes things such as flammability, acidity, alkalinity, being poisonous, etc.
How do you know when a chemical change has occurred?
• What evidence can you look for?
• The new substance starts fizzing or bubbles.
• The new substance heats up without external help.
• The new substance gets cold without you adding ice to it.
Chemical change = Chemical reaction
Examples of chemical properties
•
The flammability of paper, gasoline, alcohol.
•
The way iron rusts when left outside.
•
The way copper tarnishes to green over time
(statue of liberty).
•
The way apples, bananas, and avocadoes turn brown when sitting out in the air.
Used to identify matter without changing the substance.
Density, melting point, boiling point, freezing point, state of matter (S,L,G,P).
Things that you can see, hear, smell, touch, taste, size, shape, color, odor, weight, mass, texture, sweetness.
Physical
Properties
Used to identify matter
Chemical
Properties
How matter interacts with other matter.
Can be identified only after a change has occurred in the substance. Such as:
Acidity, alkalinity, ability to rust, ability to tarnish, ability to burn.
Energy
• The ability to cause change
• Example: “I get lots of energy from a chocolate chip cookie.”
States of energy
• Potential Energy – energy that is stored in an object because of its position or chemical composition.
• Examples:
• P- a rock sitting on a ledge
• P- a rollercoaster car at the top of the track
• C- a battery waiting to be used
• C- a can of gasoline
• Kinetic Energy- energy that is being used in the motion of something.
• Examples:
• A rock rolling down a hillside
• A rollercoaster car going around the track.
• A person running around a track.
Forms of energy
1. Chemical energy- calories in food, acid in a battery, can of gasoline.
2. Solar/radiant energy(light)- sunlight, light from a bulb, glow of hot metal.
3. Heat/thermal energy- from friction, from steam, from hot stove.
4. Sound energy-vibrations from a speaker, thunder.
5. Electrical energy- lightening, current in the wires.
6. Mechanical energy- motion of machine parts, motor, or engine.
7. Nuclear energy- nuclear fission reactions like at a nuclear power plant, nuclear fusion reactions like inside a star.
How an Electrical Power Plant Works
1. We use the chemical energy in a fossil fuel (coal or oil) to make heat.
2. The heat is used to make steam.
3. The steam is turned into mechanical energy in the turbo generator.
4. The mechanical energy is changed into electrical energy in the turbo generator.
Sources of Electric Power
1.
Fossil fuels – oil or coal is burned to heat water that turns into steam, that spins the turbine, and runs the generator. can be built anywhere. cause air pollution and there is a limited amount of them
2.
Hydroelectric power- water rushes from behind the dam and spins the turbine to run the generator. No air or water pollution. not all places have deep enough rivers.
3.
Nuclear power- heat from nuclear radiation from the reaction boils water and the steam runs the generator. can be built anywhere, no smoke or gases into the air. radiation, and it requires a lot of water that becomes unusable.
4.
Geothermal- steam from a hot spring runs the generator. no air or water pollution. not all places have hot springs.
5.
Wind power- the wind turns the blades of the wind turbines and runs the generator. No air or water pollution. Not all places have enough wind.
6.
Solar Power- light striking the silicon cells of the panel causes a flow of electrons. No generator is needed. No pollution or shortage of sunlight. Night and cloudy days.
Force- a push or a pull with a certain strength and direction, anything that can change the motion of an object.
Examples:
• Friction
• Gravity
• Wind
• Magnetism
• Water
• Muscle power
Balanced forces are equal in strength, so they don’t cause
motion or a change in motion if the object is moving.
Unbalanced forces are NOT equal in strength and that causes motion.
It moves the object in the same direction as the stronger force.
Balanced and unbalanced Forces
• Balanced forces
• Unbalanced forces
The object moves in the direction of the strongest force.
motion no motion motion
Displacement
• The distance and direction between your starting point and your ending point.
The distance you walk on a winding path can be much greater than the displacement.
10 miles NE
Speed – displacement over time
• Distance(D) divided(/) by time(t). S=D/t. Often measured in a unit of distance per unit of time. Example miles per hour (mph).
• What is the speed of a car traveling 1000 meters in 50 seconds?
• How long would it take a meal worm to travel 5 meters across the ground if it’s traveling at 0.25 m/s?
Velocity- speed and direction
Velocity has magnitude and direction
Acceleration
• Any change in speed or direction. This includes slowing down as well as speeding up, and changing direction.
• Examples: a Car coming to a stop. A car taking off from a stop. A car turning a corner. Racing. Roller-coaster ride.
Newton’s 1
st
law
• An object at rest stays at rest and an object in motion stays in motion, unless an unbalance force acts upon it.
• This is linked to inertia (an objects resistance to a change in its motion.
• Examples:
• A truck sitting in a parking space until you start the engine and make it move.
• A train moving 100mph down a track until the brakes make it stop.
• Which object has more inertia?
10 g 50g
Newton’s 2
nd
Law
• An object moves in the same direction as a force that is pushing or pulling on it.
• The object changes direction to go with the force applied to it or changes its speed to go with the force applied to it.
• It accelerates in the same direction as the force applied on it.
• Examples:
• Earth’s gravity pulling a meteor into the atmosphere. (it speeds up as gravity pulls on it)
• A paper airplane goes in the direction you push it.
• A magnet pulling a paper clip toward itself using its magnetic force.
Newton’s 3
rd
Law
• For every action there is an equal and opposite reaction.
• Two forces are involved and the are opposite to each other
• Examples:
• A person jumping on a trampoline
• Bouncing a ball
• A rocket launching into the air
Simple machines
• Inclined Planes/Pulleys (TEKS 6.8E)
• Investigate how inclined planes and pulleys can be used to change the amount of force to move an object
• Inclined plane-