Chem 30 9.2 Alkanes
advertisement
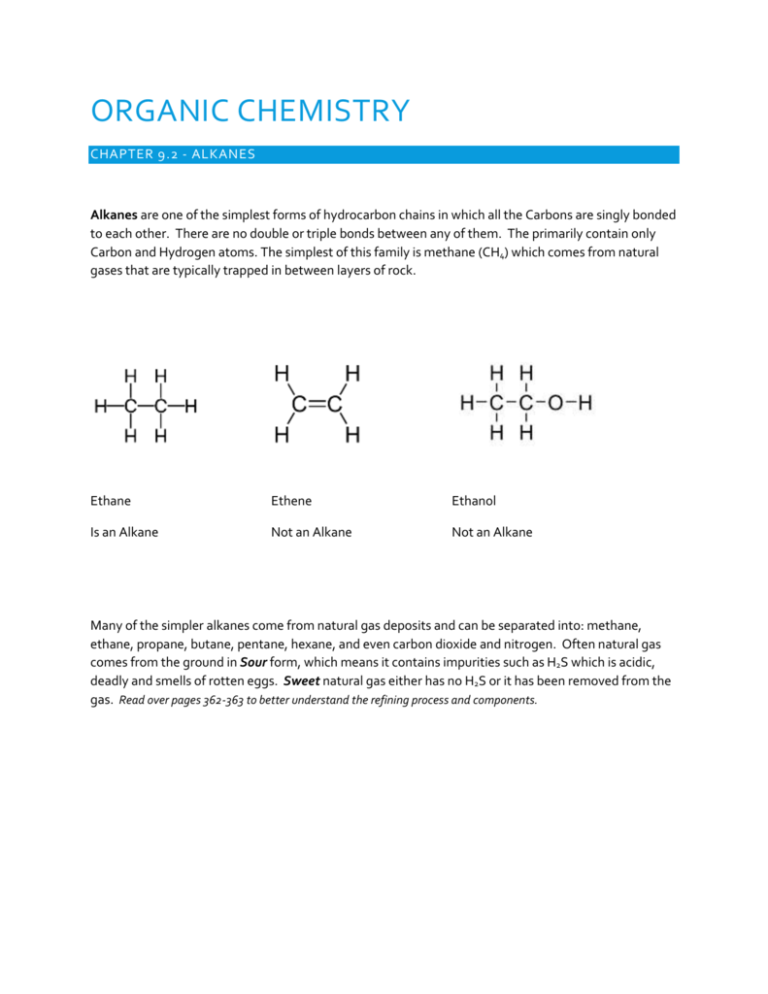
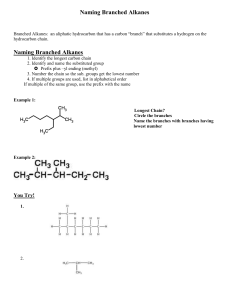
ORGANIC CHEMISTRY CHAPTER 9.2 - ALKANES Alkanes are one of the simplest forms of hydrocarbon chains in which all the Carbons are singly bonded to each other. There are no double or triple bonds between any of them. The primarily contain only Carbon and Hydrogen atoms. The simplest of this family is methane (CH4) which comes from natural gases that are typically trapped in between layers of rock. Ethane Ethene Ethanol Is an Alkane Not an Alkane Not an Alkane Many of the simpler alkanes come from natural gas deposits and can be separated into: methane, ethane, propane, butane, pentane, hexane, and even carbon dioxide and nitrogen. Often natural gas comes from the ground in Sour form, which means it contains impurities such as H2S which is acidic, deadly and smells of rotten eggs. Sweet natural gas either has no H2S or it has been removed from the gas. Read over pages 362-363 to better understand the refining process and components. After gas is pumped from the production wells water and the liquid hydrocarbons are removed. Then through a process of chemical refining in the absorption tours (processing plant) the gas is turned from sour to sweet by removing the H2S and CO2. The H2S subsequently is refined changing to SO2 then to S8 and H2O. The large amounts of yellow sulfer can then be used in other industrial practices. After the gas is turned sweet it can be refined into more specific purposes such as methane, ethane and propane. Practice problems page 364 #1,2,3,4,6 CHAPTER 9.2 – NAMING ALKANES Alkanes are a form of organic compound that contains sequences of reoccurring structure, built from adding additional -CH2 segments. This is often referred to as a homologous series. Alkanes fit the general equation CnH2n+2, which can be seen in the formulas CH4, C3H8, C8H18….etc. Alkanes, since they are formed exclusively from carbons attached only by single bonds have the greatest capacity to hold hydrogen atoms. This makes them referred to as saturated hydrocarbons. Alkanes: All bonds between carbons are single bonds Require a very organized method of naming due to the many different ways they can be combine, especially as you add more Carbons to the chain. ***NOTE: all carbons must get 4 bonds, all Hydrogens 1 and all Oxygens 2. Simplest (methane) +one CH2 group (ethane) +one CH2 group (propane)….. Memorize the prefixes for 1-10 in molecular IUPAC naming: Identification of Structural Isomers: A structural isomer is a hydrocarbon that has the same number of pieces of C and H, but arranged into different orders. This rearrangement creates entirely new compounds with entirely new properties. Therefore, when naming, it must be very clear how the compound is arranged in the naming. Drawing carbon chains. 3 standard models: 1. Structural formula 2. Condensed structural formula 3. Line structural formula Rules for naming: 1) Find the longest continuous carbon chain to get the prefix (meth-, eth-, prop-, etc) 2) As long as all the bonds between the carbons are single, then the ending is –ane 3) If there is a branch off the main chain it must also be named. This is called an Alkyl group. A one carbon long alkyl group is methyl, a two carbon long one is ethyl and a three carbon one is a propyl. The position and the number of these branches must be clear as they can also create different isomers. a. If there is only one branch no prefix is needed in front of the alkyl. If there are two or more…(di-, tri-, tetra-, etc) b. The position of the branches must be named as well by putting a number in front of the Alkyl prefix. The numbers should always start as low as possible, so check both directions on the chain. c. If there is more than one type of branch (ex a methyl and an ethyl) they are placed alphabetically. Formula: postion branch prefix-ane Ex: 2,3 dimethyl pentane Ex: 4 ethyl 3 methyl heptane Practice: Page 370 #7,8,9,10,11 Try these: Cycloalkanes In a cycloalkane, rather than a carbon chain, it makes a loop. These isomers may have the same number of carbons and hydrogens as other molecules, but the shape will give them different properties than the other isomers. General equation then becomes CnH2n, since the carbon at the ends will bond to each other removing two spaces for the hydrogens. So cycloalkanes have 2 less hydrogens than regular alkanes and a formula closer to that of alkenes which we cover in the next section. cyclopropane cyclobutane cyclohexane Methyl cyclobutane **since there are no starting points on the loop, the branches are numbered to have the smallest values. Thus, 1’s are not needed. 1,3 dimethyl cyclobutane However, even these loops can have structural isomers such as above. 1,1 dimethyl cyclobutane Try these: Page 372: # 2,4,5,6,7,8