File
advertisement
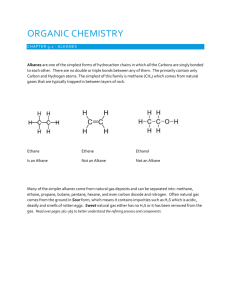
Organic Chemistry When you hear the word “organic” what comes to mind? What is organic chemistry? *Mostly found or produced in nature or any living things (biomolecule) *Usually a chain or rings of carbon atoms *Typically carbon bonding to itself, and other non-metals (H2, N2, O2, S and halogens) Carbon Bonding How many valence electron for a carbon? * # valence electrons allow for a very stable structure. *Most basic is a hydrocarbon (ex. ____NAME__ CH4 with a tetrahedral shape) *Allows for variety of allotropes: (1)diamond—strong (2)graphite– soft (3)fullerenes--spherical Organic Chem Notes Chp. 20.2 *Define key vocabulary terms, simplify *For each type of alkanes, describe and sketch *Include Table 20.1 (skip the extended formula) Chp. 20.3-20.4 *Include Table 20.2 *Simplify and paraphrase rules for naming alkanes pg. 643 *Read the section, answer #4 and 5 pg. 648 Front Alkanes ---simplest hydrocarbons, saturated Formula: C-C (single bond) Suffix: –ane Inside Example: Ethane 1 2 3 4 5 6 7 8 9 10 Meth Eth Prop But Pent Hex Hept Oct Non Dec Based on the table above, draw a methane and an ethane compounds. Using the same table, create a propane structure. Alkene Front Alkyne Formula: C=C (double bond) Suffix: –ene Example: Butene Inside -Formula: C=C (triple bond) Suffix: –yne Example: Hexyne Attachments or Side Chains # of Carbons HALOGENS Methyl 1 Ethyl 2 Propyl 3 Butyl 4 Cl Br F I ALKYLS NAME Chloro Bromo Fluoro Iodo Rules for Naming Alkanes Find longest chain write name # parent C- chain lowest # for the attachment Name attachment (see alkyls or halogen table) Indicate which Carbon (#) attachment is found Multiple attachments that are the same type state # using prefix (ex. di, tri) 6. #-prefix-alkyl (alphabetized)-base alkane 1. 2. 3. 4. 5. #1 #3 #2 #4 3-methyl pentane 3-ethyl-2-methyl pentane 2,2,3-trimethyl butane 3,4-dimethyl hexane Alkenes and Alkynes (Chp.20.7) --- double and triple bonds in a hydrocarbon chain --- rules are similar to alkanes except: *-ene for double bonds *-yne for triple bonds *# of where multiple bonds use lowest Practice: Name the following #1 #2 ANSWER #1 3-methyl-1-butene #2 2,2-dimethyl -1-propene Alkene and Alkynes Practice Problems: Draw the structure 1.) 2.) 3.) 4.) 5.) 6.) 7. 2,5-dimethyl-3-hexene 4-methyl-2-pentene 3-methyl-1-pentyne 6-ethyl-2-octyne 2-iodo-3,4-diethyl-4-methyl-2-hexene 2-bromo-4-ethyl-5,6-dimethyl-4-octyne 4-Bromo-5-Ethyl-6-Propyl-5,8-decene CLASS ACTIVITY: Functional Groups Note Cards page 660-670 Functional Group Amines Ethers Alcohols Aldehydes Ketones Carboxylic acid Esters General Formula Examples Naming Rules/ Properties/Uses *Formula: Amines *Example: Ethylamine * Naming: suffix use --amine * Properties: Front Very similar to ammonia, reactive to water Inside *Formula: R -OH Alcohol *Example: Ethanol *Naming: suffix use --ol * Properties: product of distillation Front Inside *Formula: *Example: Methyl Propyl Ether *Naming: Ethers naming each of the two carbon groups as a separate word followed by a space and the word ether. Front * Uses: Inside Diethyl ether was once used extensively as an anesthetic O *Formula: R-C-O-R’ Ester *Example: Methyl Ethanoate *Naming: The shorter Cchain will be the side group, the longer will be the main name with suffix –oate Front Inside O *Formula: R-C-H Aldehydes *Example: Propanal * Naming: suffix use --al * Properties: Found at the end of Cchains Front Inside O *Formula: R-C-R’ Ketones *Example: 2-pentanone * Naming: suffix use --none Front * Properties: Inside Found in the middle of chain O *Formula: R-C-O-H Carboxylic Acid *Example: Pentanoic Acid * Naming: suffix use --oic * Properties: Front Inside Some common named compounds include formic acid and acetic acid CLASS ACTIVITY: Functional Group Notecards • Create the rest of the cards using the same set up for each functional group. Use your textbook for resource. (NOTE: create your cards by cutting out a printer paper into 8 equal size note cards) Per. 1-3– page 660-670 Per. 4-6—page 598-606 *Remaining Functional Groups: Alcohols/Aldehydes/Ketones/Carboxylic Acid/Esters What is an isomer? *Using your materials create a saturated (4 bonds) hexane structure. * Rearrange your hexane and create an isomer. Original stucture is called nhexane but isomers can be called isohexane Naming Alkanes: Practice Problems Pt. I: Draw the following alkanes 1.) 2.) 3.) 4.) 2,3-dimethylhexane 3-ethyl-4-dimethylheptane 5-ethyl-3-methyloctane 7-butyl-2,4-dimethyl-6-propyldecane Pt. II: Name the following alkanes 5. 6. Check Practice Problem pg. 648 4. a. 3-methylheptane b and c. 2,3-dimethylbutane d. 2,3,4- trimethylheptane 5. Isomers of C8H18 Create Flash Cards/ Playing Cards DRAW EACH ONE Methane CH4 METHYL DRAW Ethane C2H6 ETHYL DRAW Propane C3H8 PROPYL DRAW Butane C4H10 ISOPROPYL DRAW Pentane C5H12 BUTYL DRAW Hexane C6H14 ISOBUTYL DRAW Heptane C7H16 Octane C8H18 ALKANE HYDROCARBON/ SINGLE BONDS Nonane C9H20 ALKENE HYDROCARBON/ DOUBLE BONDS Decane C10H22 ALKYNE HYDROCARBON/ TRIPLE BONDS Flow Chart to determine functional groups Practice Problem: Identify what type of functional group and name the structure 1. 2. 3. 2. 4. 5. 7. 6. 8. Create functional group structures AROMATICS BENZENE RING– distinct smell/ 6carbon ring/ one one plane Other Aromatic POLYMERS • Repeating monomers or smaller subunits • Organic substances and synthetic substances What are some examples of polymers? Examples • Ex. proteins, carbohydrates, lipids, plastic, rayon, styrofoam etc. Symbol Type of material Polyethylene terephalate (PET or PETE) High density polyethylene (HDPE) Polyvinyl chloride (V or Vinyl or PVC) Low density polyethylene (LDPE) Polypropylene(PP) Polystyrene (PS) Other Article: Thirsty Polymers 1. What polymer is used in baby diapers and simulates the inside portion of cactus? What is the potential amount of water that can be absorbed by this polymer. 2. List 5 common examples of polymers? 3. Explain how this polymer is able to absorb water (include the discussion of cross-linkers) 4. If the water is a salt solution, how would this affect the absorption capability. Explain. 5. What are the other uses of this polymer (in use and in development)?