organic chemistry
advertisement

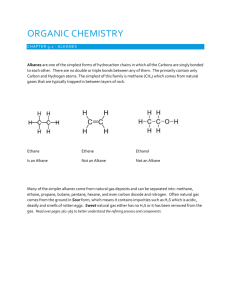
Chapter 22 Notes, part I Organic Chemistry Organic Chemistry • Chemistry of carbon containing compounds is organic chemistry. • The name is derived from the fact that early scientists thought carbon compounds could only be synthesized by organisms. Bob LOVES organic chemistry. Hydrocarbons • Organic chemistry centers around hydrocarbons— compounds made of carbon and hydrogen only. • Hydrocarbons are found all around us, especially in the petroleum industry. Bob thinks understanding hydrocarbons is a bright idea! Alkanes • Alkanes are hydrocarbons where all the carbons are attached to each other with single bonds. • A straight chain alkane has all carbons in a row (no branches). Bob jumps for joy because alkanes are grrrreat! Naming straight chain alkanes • Ten prefixes are needed for naming most common alkanes: Meth- =1 hex=6 Eth=2 hept=7 Prop=3 oct=8 But=4 non=9 Pent=5 dec=10 Now Bob is sad… …he only has six toes to count with! Naming straight chain alkanes • First, count the number of carbons in the chain. • Second, start the name with the prefix for that many carbons and end it in –ane. Branch chain alkanes and alkyl groups • Not all alkanes are in a straight line! • Anything that is attached where hydrogen is usually located is called a substituent. A hydrocarbon substituent is called an alkyl group. Name Formula • Methane CH4 • Ethane C2H6 • Propane C3H8 • • • • • • • C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 Butane Pentane Hexane Heptane Octane Nonane Decane Structure Naming branch chain alkanes • First, name the alkyl group coming off of the main chain. Give the prefix for the number of carbons, then end it in –yl. • Then name the main chain, ending just like before in – ane. Oh boy, Bob’s going to have to study this! Naming branch chain alkanes • If there is more than one place to put an alkyl group, you have to put a number before it to signify its location. • Number from the end that gives you the lowest overall number. • If there is more than one alkyl group, put a prefix to show how many there are (di, tri, tetra, etc.) Endings • Alkanes (all C-C single bonded parent chain) end in –ane – Methane CH4 – Ethane C2H6 – Propane C3H8 • Attached carbon groups (substituents) end in –yl – Methyl CH3 – Ethyl CH3CH2– Propyl CH3CH2CH2 – 3-ethylpentane Number the parent chain. • Number the parent chain so that the attached groups are on the lowest numbers Methyl is on carbon #2 of the parent chain Methyl is on carbon #4 of the parent chain 1 5 1 8 2 4 3 3 4 2 3 6 7 2 8 1 4 5 5 4 6 3 5 1 GREEN is the right way for this one! 27 Groups on 2, 3, and 5 Groups on 4, 6, and 7 1 7 2 6 3 5 4 4 Groups on 2 and 5 5 3 6 72 1 Groups on 3 and 6 Name the attached groups. • Carbon (alkyl) groups – Methyl CH3 – Ethyl CH3CH2– Propyl CH3CH2CH2 – • Halogens – Fluoro (F-) – Chloro (Cl-) – Bromo (Br-) – Iodo (I-) Designate where the group is attached to the parent chain. • Use the numbers of the parent chain from step 2 to designate the location of the attached groups to the parent chain. 2-methyl 1 2 3 4 5 Alphabetize the groups, combine like groups, and assemble. • The prefixes di, tri, tetra etc., used to designate several groups of the same kind • Prefixes are not considered when alphabetizing (Example: dimethyl = m for alphabetizing) • Parent chain goes LAST 1,1,1-trichloro-1fluoromethane 1,1-dichloro-1,1difluoromethane • 2-methylpropane • 2,3,3triMethylPentane Draw Some Simple Alkanes • 2-methylpentane • 3-ethylhexane • 2,2-dimethylbutane • 2,3-dimethylbutane Structural Formulas • “Lazy” way to write the Hydrogens • Instead of drawing the bonds, just state how many hydrogens are attached • NOTE: The bonds are between CARBONS in a parent chain, and not hydrogens! Structural Formula Lewis Structure Order of Priority • IN A TIE, halogens get the lower number before alkyl groups 4-chloro-2-methylpentane or 2-chloro-4-methylpentane? Order of Priority • IN A TIE between SIMILAR GROUPS, the group lower ALPHABETICALLY gets the lower number 4-bromo-2-chloropentane or 2-bromo-4-chloropentane ? Just remember, Bob loves you!