File
advertisement

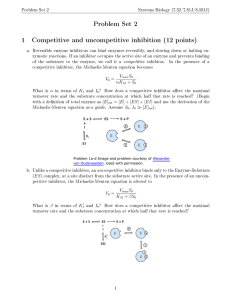
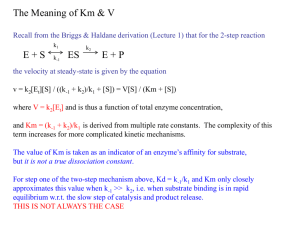
NZY NZYMES MES Amylase catalyses the breakdown of starch Amylase Starch Amylase Maltose Enzyme Substrate Enzyme Product The Lock and Key Mechanism of Enzyme Action In this diagram the enzyme breaks a large molecule into smaller ones. The same enzyme will also catalyse the reverse reaction – it will join the smaller molecules together again. The Induced Fit Hypothesis of Enzyme Action When the substrate binds to the enzyme’s active site, it ‘induces’ a change of shape so that the substrate and enzyme become fully complementary. A Graph To Show That The Activation Energy Of A Reaction Is Smaller In The Presence Of An Enzyme. DENATURATION Don’t Forget!!! Enzymes are Proteins and will show a positive result with the Biuret test! Cofactors The Effect of Temperature on the Rate of an Enzyme-Controlled Reaction The Effect Of pH The Effect Of pH on the Rate of an Enzyme-Controlled Reaction The Effect Of Changing Substrate Concentration The effect of changing the amount of substrate on the rate of an enzyme-controlled reaction. As the amount of substrate increases from 0 to B, the rate of reaction increases. As the amount of substrate increases from B to C, the rate of reaction does not increase any further. Competitive Inhibitors Non-Competitive Inhibitors (ALLOSTERIC SITE) The Effect of a Non-Competitive Inhibitor The effect of a non-competitive inhibitor on the rate of an enzymecontrolled reaction. The rate of reaction is always lower with a noncompetitive inhibitor, however much substrate is present. End-Point Inhibition By acting as an inhibitor, the end-product of a series of reactions prevents its own production. End-Point Inhibition continued…