Topic 1.4 Enzymes – Page 2
advertisement

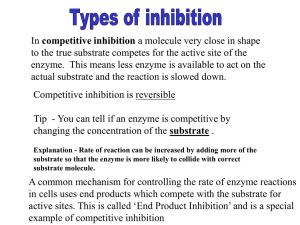
AS Unit 1: Basic Biochemistry and Cell Organisation Name: Date: Topic 1.4 Enzymes – Page 2 l. Introduction to Enzymes Completed 1. 2. 3. Go through the PowerPoint on Enzymes Read the following: Rowlands p45-50 Toole p35-44 Notes on Enzyme Inhibition Complete the past paper questions at the end of the notes Extra reading Inhibitors Affect Enzyme Activity Enzyme Control of Metabolic Pathways Enzyme Inhibition Enzyme inhibitors are molecules that bind to enzymes and decrease their rate of reaction or may even stop it completely. They achieve this by affecting the active site so that less enzyme-substrate complexes form or can no longer form. Many pharmaceutical drugs are enzyme inhibitors. There are two main categories of inhibitor: competitive inhibitor and non-competitive inhibitors. Competitive Inhibitor Enzymes are globular proteins with an active site that has a specific 3D shape. Competitive inhibitors have a similar shape to the substrate and they compete for the active site (note they only have a similar shape NOT the same shape or it would be the normal substrate molecule!). The greater the similarity of structure the inhibitor has with the normal substrate the more effective it will be as an inhibitor. Competitive inhibitors prevent the substrate binding and therefore lower the number of Enzyme Substrate complexes that can be formed. An example of a competitive inhibitor is shown below: In the example above identify the following: Enzyme:_______________________ Substrate:__________________________ Product:_______________________ Inhibitor:___________________________ Below are some examples of other competitive inhibitors. Highlight the similar structures that both the substrate and competitive inhibitor share. As you can see the shape of the competitive inhibitor has similarities in structure with the normal substrate and so can therefore fit into the active site and prevent an enzyme substrate complex forming. If the concentration of substrate is increased in a mixture of enzyme, substrate and competitive inhibitor then the level of inhibition is decreased. If the substrate concentration is increased then the substrate can compete more effectively for the active site and the relative effect of the inhibitor decreases. Interesting example of competitive inhibition – Overcoming Alcoholism ‘Antabuse’ is a medication used to stop people drinking and help them to stay off alcohol. It is administered as a daily pill, so its efficacy relies on the patient’s own motivation – if they stop taking it they can drink again. Normal metabolism of ethanol (alcohol) Antabuse competes with the aldehyde oxidase and prevents acetaldehyde from being converted to acetic acid. A build up of acetaldehyde follows, resulting in a strong feeling of nausea and other strong hangover symptoms. Non-Competitive Inhibitors Non-competitive inhibitors bind to an allosteric site or a site away from the active site. When the non-competitive inhibitor binds it causes a change in the shape of the active site. This means that the substrate can no longer bind with the active site and there are fewer enzyme substrate complexes can be formed. If the concentration of non-competitive inhibitors is high enough then the reaction can be stopped completely. Increasing the substrate concentration does not alter the rate because the inhibitor is not competing with the substrate for the active site. Increasing the concentration of the inhibitor the rate of reaction decreases. This is because there are fewer functional active sites available for reaction. Non-competitive inhibitors may be irreversible (their effects are permanent, they cause permanent distortion of the active site, see cyanide on the next page. They may also be reversible their effects are only temporary – this type of non-competitive inhibitor tends to be involved in the regulation of metabolic pathways. Metabolic pathways can be controlled by something called end-product inhibition. If a metabolic pathway becomes too active i.e. it is producing too much end product for the cell to deal with, the end product inhibits one of the enzymes that catalyses an early step in the metabolic pathway so less product is formed. Tryptophan is an essential amino acid for humans, we cant produce it so we must consume it in our diet. E.coli bacteria can produce this amino acid when needed. However if they are in a tryptophan rich medium or they have produced a high level of tryptophan, the tryptophan will act as end product inhibitor. This helps to conserve energy – it will not be wasted on excess production. When tryptophan concentration declines the level of inhibition will decline, the metabolic pathway will resume. Cyanide a Criminally Interesting Non-Competitive Inhibitor Cyanide is a famously fast-acting poison due to its ability to induce extreme chemical suffocation of cells and to disrupt enzymatic processes. It’s probably most lethal in the gaseous form of hydrogen cyanide (HCN), which the Nazis infamously employed in their concentration camp gas chambers. At a high dose, about 3000 ppm in the air, it can kill in one-to-three minutes. When we swallow cyanide, it’s usually as one of the two cyanide salts, potassium cyanide (KCN) and sodium cyanide (NaCN). Sodium cyanide is slightly more toxic but either of these will kill in a trace amount – somewhere between 100-300 milligrams – last year an ex-banker, shortly after being convicted of malfeasance, killed himself with potassium cyanide. So cyanide is fast and it is reliably lethal. It was the poison found in the body of a murdered Chicago lottery winner. There’s a history – usually dated back to World War II – of spies carrying suicide pills, just in case they were captured. These were reportedly called L-pills (L for Lethal). The International Spy Museum, in Washington D.C., displays eyeglasses that contained a tiny L-pill compartment – the captured spy could supposedly casually chew on the temple (or arm) of the glasses and release the poison. The Encyclopedia of Espionage cites the case of a CIA mole in the Soviet Union, who eluded prison or worse by biting the cyanide loaded tip of a fountain pen. The Biology of Cyanide Cyanide is an irreversible enzyme inhibitor. Cyanide ions bind to the iron atom of the enzyme cytochrome c oxidase (also known as aa3) in the fourth complex in the mitochondrial membrane in the mitochondria of cells. This denatures the enzyme, and the final transport of electrons from cytochrome c oxidase to oxygen cannot be completed. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected. 1. 3.