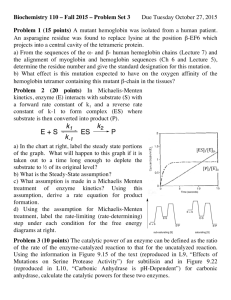
1/(V/Km)
advertisement

The Meaning of Km & V Recall from the Briggs & Haldane derivation (Lecture 1) that for the 2-step reaction E+S k1 k-1 ES k2 E+P the velocity at steady-state is given by the equation v = k2[Et][S] / ((k-1 + k2)/k1 + [S]) = V[S] / (Km + [S]) where V = k2[Et] and is thus a function of total enzyme concentration, and Km = (k-1 + k2)/k1 is derived from multiple rate constants. The complexity of this term increases for more complicated kinetic mechanisms. The value of Km is taken as an indicator of an enzyme’s affinity for substrate, but it is not a true dissociation constant. For step one of the two-step mechanism above, Kd = k-1/k1 and Km only closely approximates this value when k-1 >> k2, i.e. when substrate binding is in rapid equilibrium w.r.t. the slow step of catalysis and product release. THIS IS NOT ALWAYS THE CASE Enzyme Inhibitors in Steady-State Kinetics -- Drugs, poisons, mechanistic probes -- Reversible, irreversible, suicide -- Competitive, noncompetitive, mixed, uncompetitive -- Product inhibition -- Substrate inhibition -- Transition state analogs On-Line References for Steady-State Enzyme Kinetics Dr. Peter Birch, University of Paisley http://www-biol.paisley.ac.uk/kinetics/contents.html University of Texas http://www.cm.utexas.edu/academic/courses/Fall2001/CH369/LEC05/Lec5.htm Terre Haute Medical College http://web.indstate.edu:80/thcme/mwking/enzyme-kinetics.html Reversible Inhibitors: E+I EI Fast acting. Generally non-covalent EI complex. Removal restores enzyme activity. Irreversible Inhibitors: E+I EI Often slow, time-dependent inactivation. Often covalent EI complex. Enzyme permanently disabled. Suicide Inhibitors: E + I* EI* EI Enzyme converts precursor into irreversible inhibitor. Competitive Inhibitors 1. Competitive Inhibition by Active Site Binding -- reversible -- inhibitor (usually) structurally similar to substrate -- inhibitor competes directly for substrate binding to active site (mutually exclusive binding) -- effects can be overcome by increasing substrate concentration QuickTime™ and a GIF decompressor are needed to see this picture. Competitive Inhibitors 2. Competitive Inhibition by Conformational Change -- reversible -- substrate and inhibitor may be dissimilar -- inhibitor binds to remote site on enzyme, but causes a conformational change that precludes substrate binding to the active site -- likewise, substrate binding to active site causes a conformational change that precludes inhibitor binding to its site -- binding is still mutually exclusive -- effects of inhibitor can still be overcome by increasing substrate concentration Kinetics of Competitive Inhibitors Remember: Inhibitor & substrate binding are mutually exclusive, also rapid & reversible. High [I] competes out substrate, so enzyme is almost completely inhibited. High [S] competes out inhibitor, so enzyme is almost fully active. E+S ES E+I EI EI + S EIS ES + I EIS Effect on Km. -- Km is an indicator of enzyme-substrate affinity (like a dissociation constant). -- With inhibitor present, both free enzyme (E) and EI complex exist. -- E has normal affinity for S; EI has no affinity for S. -- Solution average affinity decreases, therefore Km increases. Effect on V. -- V is the velocity at very high [S]; i.e., conditions that compete out inhibitor. -- Thus V is unchanged. Kinetics of Competitive Inhibitors Effect on V/Km. --V/Km is the rate constant at low [S]. Why? At [S] << Km, the Michaelis-Menten equation simplifies from v = V[S] / (Km + [S]) to: v = (V/Km)[S] = k[S] E+S ES E+I EI EI + S EIS ES + I EIS Slope = V/Km v Anything that affects V or Km affects V/Km. + inhibitor -- Km increases, V unchanged. -- Therefore V/Km decreases. [S] Effects of Competitive Inhibitor on Lineweaver-Burk Plot 1/v = (Km/V)(1/[S]) + 1/V = Km/V, i.e. reciprocal of rate constant V/Km Non-Competitive / Mixed Inhibitors -- Binds to site on enzyme remote from active site. -- Causes conformational change in enzyme that prevents conversion of substrate to product, but does not prevent substrate binding to enzyme. -- I, S binding is not mutually exclusive. -- Comes in 2 varieties: Classic & Mixed 1. Classic Non-Competitive Inhibitors (Rare). -- do not alter affinity of substrate binding. 2. Mixed Inhibitors (Common). -- typically lower the affinity of substrate binding. Kinetics of Non-Competitive / Mixed Inhibitors Remember: Inhibitor & substrate binding are NOT mutually exclusive. E+S ES EIS complex forms by either of two routes, but cannot convert substrate to product. E+I EI Substrate cannot compete out the inhibitor, so inhibitor works well at low and high [S]. ES + I EIS EI + S EIS Effect on Km. -- CLASSIC Non-Competitive Inhibitor: no effect on substrate affinity; Km unchanged. -- MIXED Inhibitor: allows substrate binding but lowers affinity; Km increases. Effect on V. -- Both CLASSIC & MIXED inhibitors work at high [S], so V decreases. Effect on V/Km. -- Both CLASSIC & MIXED inhibitors also work at low [S], so V/Km decreases. Effects of CLASSICAL Non-Competitive Inhibitor on Lineweaver-Burk Plot 1/v = (Km/V)(1/[S]) + 1/V = Km/V, i.e. reciprocal of rate constant V/Km Effects of MIXED Inhibitor on Lineweaver-Burk Plot 1/v = (Km/V)(1/[S]) + 1/V = Km/V, i.e. reciprocal of rate constant V/Km Uncompetitive Inhibitors -- Cannot bind to free enzyme. -- Binds only to enzyme-substrate complex (ES). * substrate binds directly to inhibitor, or * substrate induces conformational change required for inhibitor binding. -- S, I binding is not mutually exclusive, it is required. -- Once bound, inhibitor prevents enzyme from converting substrate to product. Kinetics of Uncompetitive Inhibitors Remember: For uncompetitive inhibitor to work, FIRST substrate must bind to enzyme THEN inhibitor must bind to ES complex. Inhibitor binding to free enzyme is not allowed. Uncompetitive inhibitors are not effective at low [S], because most of the enzyme exists as free enzyme. They are effective at high [S] because most of the enzyme exists as ES complex. E+S ES ES + I EIS E+I EI Effect on Km. -- Inhibitor binding to ES complex draws E + S <-> ES binding equilibrium to right via Law of Mass Action, thereby increasing the apparent affinity of enzyme for substrate, so Km decreases. Effect on V. -- Inhibitor is most effective at high [S] where lots of ES complex forms, so V decreases. Effect on V/Km. -- Inhibitor is least effective at low [S] where there is little ES complex, so V/Km is unchanged. (decrease in Km balances decrease in V, so ratio is insensitive to inhibitor) Effects of Uncompetitive Inhibitor on Lineweaver-Burk Plot 1/v = (Km/V)(1/[S]) + 1/V = Km/V, i.e. reciprocal of rate constant V/Km Analysis of Inhibition Constants Consider the following schematic for enzyme binding to substrate and inhibitor: E KS 1 KI 2 EI ES P 3 KI’ 4 KS’ EIS P 1 2 3 4 Always possible Not possible with uncompetitive inhibitors Not possible with competitive inhibitors Not possible with competitive or uncompetitive inhibitors A noncompetitive inhibitor is capable of all four reactions, but the classical noncompetitive inhibitor, as opposed to a mixed one, is a special case. With these inhibitors Ks (of which Km is usually a squishy approximation) and Ks' are equal to each other, as are Ki and Ki'. Using MIXED INHIBITION as an example, we’ll consider 3 different ways to estimate Ki values: -- calculation -- use of secondary plots -- Dixon plots Calculation. Please note: equations on Paisley website are incorrect!! Interconversions between apparent Km and V values (those observed in presence of inhibitor) and the true values involve multiplication or division by the term (1 + i/Ki) or (1 + i/Ki’), where i = free inhibitor concentration. * Substitute these terms into M-M equation to derive full velocity equations for each inhibition model. Cornish-Bowden (1979) Enzyme Kinetics, Butterworth & Co., London, p. 79 Primary Plot: Lineweaver-Burk Plots of kinetics experiments performed at multiple, fixed concentrations of a MIXED-type non-competitive inhibitor. V/Km decreases V decreases Km increases Secondary Plot #1: 1/Vapp vs. Inhibitor Concentration MIXED non-competitive Vapp = V / (1 + i/Ki’) rearranges to: 1/ Vapp = (1/V Ki’)i + 1/V - Secondary Plot #2: 1/(V/Km)app vs. Inhibitor Concentration MIXED non-competitive = Kapp / Vapp Vapp/Kapp = (V/K) / (1 + i/Ki) rearranges to: Kapp/Vapp = (K/VKi)i + K/V - Secondary plots with different inhibitor types The sample plots shown here were produced using a mixed inhibitor, as this is the kinetically most complex of the types that we've studied. For the other types matters are simplified as follows: * Classical noncompetitive inhibitor * The secondary plots are made as above but the two plots should give identical results as Ki and Ki' are equal for these inhibitors. * Competitive inhibitor * The first secondary plot cannot be made as there is no change in maximal velocity. This plot is not required though as it gives Ki' which is irrelevant for a competitive inhibitor. * Uncompetitive inhibitor * This is really the opposite of the competitive inhibitor. The second secondary plot can't be made as there is no change in slope. Again this is not required as the Ki is irrelevant to an uncompetitive inhibitor. Use of Dixon Plot to Estimate Ki of Inhibitor -- velocities measured at muliple fixed substrate concentrations, inhibitor concentration is varied. -- graph of 1/v vs. i gives intersecting lines; Ki is derived from the point of intersection. * Classical NC: all lines intersect on horiz. axis --> read -Ki directly * Mixed or Competitive: lines intersect above horiz, axis; drop a line to -Ki * Uncompetitive: lines are parallel, cannot calculate Ki which is irrelevant anyway. Restrictive: cannot Calculate Km, V, or Ki’ Mathematical Basis of Dixon Plot for Mixed Inhibition Cornish-Bowden (1979) Enzyme Kinetics, Butterworth & Co., London, p. 81 Product Inhibition EP E+P Products bind to the enzyme active site using the same bonds, or at least a subset of the bonds, used to bind substrate. Frequently products are capable of binding to free enzyme, and do so rapidly and reversibly. -- for a single-substrate enzyme, this can lead to competitive inhibition since substrate and product binding are mutually exclusive. -- product inhibition is more complicated in multi-substrate enzymes. Use of initial velocities avoids the effects of product inhibition on kinetics. (but see Single Progress Curve method) Occasionally product release is slow and can limit the catalytic turnover of an enzyme. In this case there is usually some kind of exchange factor requirement. Product inhibition can be a useful tool for understanding enzyme kinetics, especially of multi-substrate systems. Substrate Inhibition (a.k.a. Excess Substrate Inhibition) An odd kind of kinetic behavior in which velocities actually decrease, rather than continue to approach V asymptotically, at high substrate concentrations. This phenomenon can seriously complicate the analysis of kinetics data. Seems to be most common in enzymes with large and complex substrates, such as nucleic acids, polysaccharides, etc., but is probably over-reported. Hypothetical V When you see something like this, first check for problems/artifacts with your assay. -- substrate or enzyme precipitation st high [S]. -- metal ion chelation. -- pH or ionic strength changes, etc. If you can eliminate all of the likely systematic errors and artifacts, then you might need to consider substrate inhibition in your kinetic model. How Does True Substrate Inhibition Occur? Example: Invertase, a.k.a. b-fructofuranosidase Catalyzes: sucrose (disccharide) + H2O --> glucose + fructose (monosaccharides) Substrate inhibition of invertase probably occurs when 2 molecules of substrate (sucrose) bind to the active site simultaneously, in an improper end-on fashion. Each sucrose molecule blocks the other from assuming the correct position in the active site that leads to catalysis. For inhibition to occur, the binding of the second substrate molecule must follow very rapidly upon binding of the first, otherwise the first substrate would be hydolyzed. This is only likley to occur at very high [substrate]. Can you think of other ways that substrates could inhibit their enzymes? Determining Kinetic Parameters When Substrate Inhibition Occurs -- Substrate inhibition introduces curvature at the lower end of a Lineweaver-Burk Plot. You wouldn’t want to use v4 weighted linear regression here! -- You can still extrapolate to slope and intercept using low [S] data, but recall that this data contains the most error. Rate Equations Considering Substrate Inhibition Direct fitting of v vs. [S] curve is potentially another way to extract kinetic parameters from substrate-inhibited enzymes. Cornish-Bowden (1979) Enzyme Kinetics, Butterworth & Co., London, pp. 93-94 Siesta Time!