Haematology in the ICU
advertisement

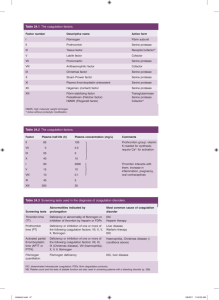
HAEMATOLOGY IN THE ICU – TAKE 2 Bryony Ross 22/2/2010 Topics Anaemia Thrombocytopaenia DIC/TTP/HUS HITTS – Blood products and their use Selected Recombinant products Coagulation Interpretation of investigations Common causes of deranged coags Tips and tricks Anaemia Develops in almost all patients in ICU for prolonged periods Patients on mechanical ventilation receive ~75% of all red cell transfusions Usually multifactorial erythropoietin production and blunted response Bleeding Frequent phlebotomy Anaemia – investigation of cause Simply, production loss destruction of red cells Exclude ongoing bleeding in surgical and trauma patients Consider haemolysis Particularly in the transfused patient (transfusion associated haemolysis) Bilirubin, reticulocyte count, LDH, haptoglobins, characteristic blood film Thrombocytopaenia Plt count <100 ~40% Plt count <50 ~ of ICU patients 10-20% of ICU patients EXTENSIVE causes Again, usually multifactorial Thrombocytopaenia – pt evaluation History of prior thrombocytopaenia and setting in which it occurred Underlying marrow disease and preexisting morbidities that can induce chronic thrombocytopaenia Liver disease (and Etoh intake) Neoplasia ITP The blood film EDTA clumping (pseudothrombocytopaenia) Exclude by repeating test with citrate tube (note: can’t be added on if coags have already been performed on the sample) Schistocytes underlying thrombotic microangiopathy TTP/HUS/DIC Poikilocytes or nucleated RBC Myelophthisic process Abnormal leukocytes Malignancy, myelodysplasia, or syndrome of congenital thrombocytopaenia Thrombocytopaenia - infection Common in critically unwell patients DIC “Endothelial damage syndrome” – eg meningococcus, pneumococcus Platelets clump and block capillaries and platelet consumption Enhanced clearance of platelets coated by antiplatelet antibodies or nonspecifically bound immunoglobulin Accelerated platelet phagocytosis induced by concentrations of macrophage colony-stimulating factor Infection of bone marrow stromal cells and megakaryocytes with viruses Treat underlying infection and plt transfusion Aim for plt count 15-20 Thrombocytopaenia – massive transfusion Transfusion of more that 15-20 units of RBC can lead to dilutional thrombocytopaenia Hypothermia, platelet dysfunction and dilutional coagulopathy (if not properly treated) will also lead to bleeding in the massively transfused patient. Levy JH. Massive transfusion coagulopathy. Sem Hematol.2006;43:S59–63. Drug induced thrombocytopaenia Diagnosis of exclusion – need temporal relationship, usually resolves about 7-10 days after cessation of drug heparin – discuss separately Variety of mechanisms Usual offenders trimethoprim/sulfamethoxazole, beta-lactam antibiotics, vancomycin, cephalothin, carbamazepine, hydrochlorothiazide, nonsteroidal antiinflammatory drugs, phenytoin, procainamide, quinidine and quinine, rifampin, sulfasalazine, sulfonylureas, and valproic acid. Heparin induced thrombocytopaenia Most common cause of drug-induced, antibodymediated thrombocytopaenia 1-2 % of pts on heparin with develop isolated thrombocytopania (HIT) In ~30 of those patients, thrombocytopaenia is accompanied by thrombosis (HITT) Both conditions are 5-10 times more likely in patients treated with UFH vs LMWH Neither condition has been reported with fondaparinux HITT Clinical diagnosis Consider in patients with an otherwise unexplained fall in plt count of at least 50% occurring 5-14 days after starting heparin NOTE: with recent heparin exposure (within the preceding 36 months, HITT can occur within a much shorter timeframe (median 10.5 hours) Several HITT screening questionnaires available, which indicate the pre-test probability prior to blood investigations (ask the Haem reg) Pre-test probability is extremely important when interpreting results HITT Thrombocytopaenia is the lesser concern Bleeding is very uncommon Thrombosis is often severe and life-threatening Venous thrombi more common except in pts with underlying arterial vascular disease Mortality ~20% Limb amputation ~10% 50% of patients will develop thrombosis on cession of heparin if alternate anticoagulation is not initiated HITT HITT screen Immunologic measurement of antibodies against heparinPF4 complexes or the ability of such antibodies to activate platelets Alternate anticoagulation Lepirudin Intravenous infusion, Monitored using APTT Cease warfarin Talk to Haematology Warkentin TE. An overview of heparin-induced thrombocytopenia syndrome. Sem Thromb Haemost. 2004;30:273–83 Aster RH. Drug-induced immune cytopenias. Toxicology.2005;209:149–53. Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101:502–7. Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–92. Thrombocytopaenia – TTP/HUS TTP and HUS Thrombotic microangiopathies associated with microangiopathic haemolytic anaemia and thrombocytopaenia Both can be associated with neurologic abnormalities, renal dysfunction and fever TTP usually has incidence of neurologic manifestations HUS usually has incidence of renal dysfunction Generally, microangiopathic haemolytic anaemia and thrombocytopaenia without another apparent cause is sufficient criteria to start treatment HUS 2 main variants Most common is following VTEC with abdominal pain and bloody diarrhea ~ 20% of patients progress to HUS and ARF within 5-6 days Most common in paediatrics and in epidemics Second is in post-partum period Also familial form associated with deficiency of complement factor H HUS not usually associated with ADAMTS13 Does not respond as well to plasma exchange TTP Pathogenesis unclear, may involve deficiency of vWFcleaving protease (ADAMTS13) leading to in ultralarge vWF multimers that bind to platelets and induced agglutination ADAMTS13 deficiency is most often due to antibodies against the protease HAPS is the only lab in NSW that offers ADAMTS13 testing However, ADAMTS13 can also occur in liver disease, pregnancy and DIC Levels in these conditions are usually about 5% and levels below this range appear to have high specificity for TTP TTP Fatal in >90% of cases if untreated Plasma exchange induces remission in ~85% of patients Corticosteroids controversial ~30% of cases will relapse within 12 months, and some pts relapse multiple time Splenectomy can be useful to relapse Rituximab can be used for refractory cases George JN. How I treat patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Blood.2000;96:1223–9. McCrae KR, Sadler JE, Cines DB. Thrombotic thrombocytopenic purpura and the hemolytic uremic syndrome. In: Hoffman R, Benz EJ Jr, Shattil SJ, et al., eds. Hematology: Basic Principles and Practice. Philadelphia: Elsevier, Churchill, Livingstone; 2005:2287–304. Sadler JE, Moake JL, Miyata T, et al. Recent advances in thromboticthrombocytopenic purpura. Hematology: ASH Education Program Book. Washington DC: American Society of Hematology; 2004:407–23. Thrombocytopaenia – catastrophic antiphospholipid antibody syndrome Rare Characterized by multiorgan involvement by microthrombi and thrombocytopaenia Only 15% have shistocytes on film Guidelines for diagnosis Lupus anticoagulant +/- antiphospholipid antibodies Involvement of 3 or more organs Development of manifestations within 1 week or less Confirmation of histopathology of small vessel occlusion in at least one organ Treat with plasma exchange, aggressive anticoagulation and antibiotics DIC DIC DIC results from the disordered regulation of normal coagulation Excess thrombin generation with secondary activation of the fibrinolytic system. Uncontrolled thrombin and plasmin generation results in consumption of clotting factors and proteolysis of platelet membrane glycoproteins. DIC is triggered by diseases that promote the expression of TF, which then complexes with factor VII to initiate coagulation TNF, IL-1, and neutrophil elastase all damage the endothelium, causing the expression of TF. Other sources of TF include damaged cerebral tissue; promyelocytic, myelomonocytic, and monocytic leukemia cells; and placental tissue substances associated with obstetric catastrophes. Cysteine proteases and proteases derived from mucin- producing adenocarcinomas or snake venoms can also directly activate coagulation factors to induce DIC. Acute hemolytic transfusion reactions promote DIC indirectly through the formation of circulating immune complexes that activate complement or directly by the toxic effects of damaged erythrocyte membranes; both of these processes result in endothelial cell damage. Hypotension from any cause can result in endothelial cell damage, triggering DIC. DIC The clinical and laboratory manifestations of DIC result from the combined effects of thrombin and plasmin produced in excess of that required for normal hemostasis. Bleeding from venepuncture sites Spontaneous thrombosis Lab diagnosis Evidence of fragmentary haemolysis, fibrinogen and platelet consumption, combined with enhanced fibrinolytic activity ie fibrinogen, platelets, XDP’s, and characteristic blood film PT is usually prolonged, reflecting coagulation factor consumption APTT is variable, depending on FVIII levels TT is prolonged (interference by FDP with fibrin polymerization +/- hypofibrinogenaemia) DIC - treatment Treat underlying disease process Treat the coagulopathy that results in the thrombotic and haemorrhagic manifestions patients who are bleeding or who have thrombosis require treatment of their coagulopathy Maintain platelets >20 FFP to replace consumed coagulation factors Cryoprecipitate if fibrinogen <1.5 DIC – further treatment Failure of the plt count or fibrinogen level to increase despite vigorous replacement = ongoing consumption (common) Heparin (low doses, 10 units/kg/hr) may be used to block activation of the coagulation system, or if there is thrombosis Fibrinolytic inhibitors - ε-aminocaproic acid or transexamic acid not useful (exaggerate the thrombotic component) DIC – other treatment Use of endogenous inhibitors of coagulation as a specific therapy for severe sepsis Often complicated by DIC Recombinant APC in a 96 hr infusion was shown to improve survival in a recent trial Pts with significant coagulopathies or thrombocytopaenia were excluded Antithrombin has not been shown to be effective in improving survival with sepsis Coagulation – the basics The tissue-factor VIIa complex is the most important in vivo initiator of coagulation Coagulation – the basics TF is a transmembrane protein expressed by fibroblasts in the subendothelium During activation of coagulation in response to vascular injury, TF is expressed on the surface of monocytes and endothelial cells Coagulation is initiated when circulating FVIIa binds to TF, activating trace amounts of factor X and factor IX. After VIIa and TF bind, generation of a definitive clot requires production of small amounts of thrombin (by factor Xa) followed by further generation of thrombin (mediated by XI, VIII, and V) Large amounts of thrombin are crucial to cross-link fibrin (FXIIIa) and reduce fibrinolysis Coagulation – the basics In Vitro Coagulation - tests PT and APTT measure the integrity of the coagulation system Sensitivity of different PT and APTT reagents to deficiencies of coagulation factors or to the presence of inhibitors may vary. Ideally, results are abnormal only when a coagulation factor deficiency is severe enough to be clinically important Eg. APTT should not be sensitive to factor VIII or IX levels that are >50% of normal as A few notes on coags “coags” on a request form = PT and APTT The lab only adds on a TT if one or both is abnormal The lab will do a protamine correction if there is a suspicion of heparin contamination (ie a prolonged APTT) The lab will do a lupus anticoagulant if this is suspected (when there is a coag scientist in the lab) It is possible to have a normal PT and APTT and a fibrinogen of <1.5 Fibrinogen levels are not affected unless the sample is grossly heparin contaminated APTT > 100 A few notes on coags pH At a pH < 7.2, clotting is severely impaired If pH <7.0, clotting WILL NOT occur Treatment is to reverse the acidosis and give products as directed by APTT/PT/Fibrinogen as required. Temp has similar effect A few notes on coags Don’t forget about vitamin K in chronic malnutrition (including those with alcohol dependency) or conditions that limit absorption of dietary vitamins such as biliary obstruction, coeliac disease, ulcerative colitis, regional enteritis, cystic fibrosis, short bowel syndrome or intestinal resection (particularly of the terminal ileum, where fat-soluble vitamins are absorbed). In addition, some drugs may reduce vitamin K levels by altering liver function or by killing intestinal flora that make vitamin K Random other useful stuff Blood transfusion site on the intranet Lists all blood products available Has protocols for administration ARCBS Blood products Useful physiology stuff for exams Paul says it is more up to date than Brandis Massive Trauma What’s in the MTP MTP1 – 4 PRC, 4 FFP, 10 Cryo MTP2 – 4 PRC, 4 FFP, 1 plt Why can’t I use this outside the setting of trauma? In the absence of hypovolaemic shock and significant liver dysfunction, exchange of one circulating plasma volume does not reduce the clotting factor activities below levels necessary to maintain haemostasis (ie 50%) Use PT/APTT/fibrinogen to guide factor replacement therapy Thrombocytopaenia is the most frequent abnormality associated with massive transfusion Talk to haematology Blood products in Children Red blood cells Packed cells (mls) = wt (kg) x Hb rise required (g/L) x 0.4 Platelets 5-20ml/kg FFP 10-20 (will raise plt count by 50-100) ml/kg Cryoprecipitate 5-10ml/kg See Clinical Practice Guideline on Kaleidoscope Warfarin Reversal Guidelines Blood Products Prothrombinex Indicated in prophylaxis and treatment of bleeding in patients with single or multiple congenital deficiencies of factor II or X and in patient with single or multiple acquired prothrombin complex factor deficiency requiring partial or complete reversal (eg warfarin) Contraindicated in patients with thrombosis or DIC Blood Products Novo7 Treatment of deficiency of Factor VIIa or for treatment of massive uncontrolled bleeding Cardiac surgery, post partum haemorrhage and trauma The use of Factor VIIa in those with advanced hypovolaemic shock is futile MUST have Surgical haemostasis pH above 7.18 Temp above 35 Platelet count above 50 Adequate fibrinogen to clot (give cryo first) Last words Clexane can’t be reversed Always monitor clexane – Xa levels in renal impariment All fragments are not haemolysis Most commonly seen in renal impairment Always do an LDH and retics if you suspect haemolysis Everyone in ICU probably needs a Fibrinogen when their coags are checked Think about using Ptx for a prolonged PT/APTT in liver disease if there is bleeding or require surgery if fluid volume is an issue To assess coagulation requires APTT/PT/fibrinogen and platelet count 25 units/kg + one bag of FFP (for extra VIIa) = about 4 bags of FFP There is a finite amount of plt in cupboard – that is why you need to ask for it – we often run out and have to triage usage In desperation you can use a (well labelled) swab for a blood group. Thanks! Feel free to drop in to the lab for advice and to meet the lab staff.