Chemical Formulas and Chemical Compounds
advertisement

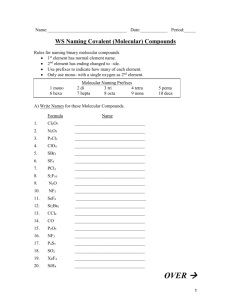
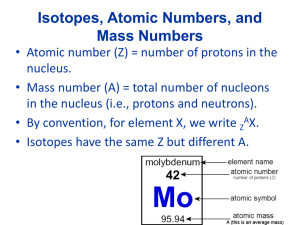
Chemical Formulas and Chemical Compounds Chapter 7 Chemical Formulas Octane Molecule C8H18 Aluminum Sulfate formula unit Al2(SO4)3 Monatomic Ions Def: ions formed from a single atom Naming ions Cation – use element name Anion – change ending of element to -ide Monatomic Ions d – block elements and others with multiple ions Use Roman numeral in name Fe+3 Iron(III) ion Fe+2 Iron(II) ion Metals always have positive charge Polyatomic Ions Polyatomic Ions NO3- Nitrate NO2- Nitrite The –ate means 1 more oxygen SO4-2 Sulfate SO3-2 Sulfite Polyatomic Ions ClO4- Perchlorate ClO3- Chlorate ClO2- Chlorite ClO - Hypochlorite Binary Ionic Compounds Def: composed of 2 elements Total (+) charge must = total (-) charge Write the formula: Mg and Cl K and N Al and O Compounds with Polyatomic Ions Iron(II) and Nitrite Boron and Perchlorate Ammonium and Oxygen Naming Ionic Compounds Name the cation first, then the anion NaOH RbCl Ag2SO3 Fe3N2 Al(MnO4)3 Writing Formulas Potassium sulfide Copper(II) sulfate Sodium carbonate Calcium chloride Naming Binary Covalent Compounds CO, CO2 We use PREFIXES in the name!! Prefixes Mono- One Hexa- Six Di- Two Hepta- Seven Tri- Three Octa- Eight Tetra- Four Nona- Nine Penta- Five Deca- Ten Covalent Naming Rules 1. Use prefixes to show how many of each element • Single atom in first element, mono is not used 2. The second element name ends in –ide 3. Drop vowel at end of prefix if element begins with vowel (except: di and tri) Examples N2O4 PCl5 Nitrogen Dioxide Boron Trifluoride Diphosphorus Pentoxide Naming Acids 2 types of acids: 1. Oxyacids: hydrogen and polyatomic ion 2. Binary Acids: hydrogen and halogen Naming Oxyacids Replace ending of anion name Anion Suffix Acid Suffix - ite - ous - ate - ic Naming Oxyacids H2SO4 anion SO4-2 Sulfuric Acid H2SO3 anion SO3-2 Sulfurous Acid HClO4 HNO3 Naming Binary Acids Hydrogen and a halogen Use prefix hydroUse suffix –ic Followed by acid Naming Binary Acids HCl HF HI HBr Formula Mass Mass of H2O? Formula Mass: mass of molecule, formula unit, or ion is sum of masses of all atoms represented (amu) Ca(NO3)2 Molar Mass Def: mass of 1 mole of compound – use molar masses of elements (g/mol) (NH4)2CrO4 Molar Mass in Conversions Remember flow chart?? What is mass (g) of 3.04 mol of ammonia vapor, NH3? How many molecules are in 4.15 x 10-5g C6H12O6? More Conversions How many H atoms are in 7.1 moles of C6H12O6? How many formula units are in 4.5 kg Ca(OH)2? What is the mass of H2SO4, if you have 1.53 x 1023 sulfate ions your compound? Percent Composition A basket of fruit = 120 g 3 apples = 50 g 2 oranges = 35 g 2 bananas = 20 g Basket = 15 g What percentage of the basket’s mass is from the apples? Percent Composition What is the percent composition by mass of each element in (NH4)2O? What percentage by mass of Al2(SO4)36H2O is water? Empirical Formula Def: formula showing smallest wholenumber mole ratio Ex: B2H6 Molecular Formula BH3 Empirical Formula Finding Empirical Formula Determine the empirical formula of the compound with 17.15% C, 1.44% H, and 81.41% F. CHF3 Finding Empirical Formula Find empirical formula of 26.56% K, 35.41% Cr, and rest O. K2Cr2O7 Finding Molecular Formula x(empirical formula) = molecular formula x(Emp.Form Mass) = Molec.Form Mass x = Molecular Mass Empirical Mass Finding Molecular Formula Determine molecular formula of compound with empirical formula CH and formula mass of 78.110 amu. Finding Molecular Formula Sample has formula mass of 34.00 amu has 0.44g H and 6.92g O. Find its molecular formula.





![Measurement of the top-quark mass in all-jets t[bar over t]](http://s2.studylib.net/store/data/011906409_1-44a321e596c67916c5e23bceae1677d0-300x300.png)
