Type 2 Diabetes Management Goals
advertisement

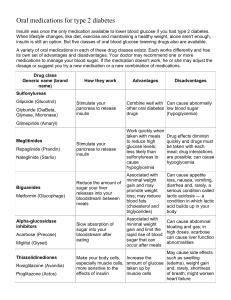
Glycemic Management in Type 2 Diabetes 1 AACE Comprehensive Care Plan Disease management from a multidisciplinary team Antihyperglycemic pharmacotherapy Comprehensive Care Plan Comprehensive diabetes self-education for the patient Therapeutic lifestyle change 2 Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. Glycemic Management in Type 2 Diabetes Therapeutic Lifestyle Change 3 Components of Therapeutic Lifestyle Change • • • • • • Healthful eating Sufficient physical activity Sufficient sleep Avoidance of tobacco products Limited alcohol consumption Stress reduction 4 Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. AACE Healthful Eating Recommendations Topic General eating habits Recommendation Regular meals and snacks; avoid fasting to lose weight Plant-based diet (high in fiber, low calories, low glycemic index, high in phytochemicals/antioxidants) Understand Nutrition Facts Label information Incorporate beliefs and culture into discussions Informal physician-patient discussions Use mild cooking techniques instead of high-heat cooking Carbohydrate Understand health effects of the 3 types of carbohydrates: sugars, starch, and fiber Target 7-10 servings per day of healthful carbohydrates (fresh fruits and vegetables, pulses, whole grains) Lower-glycemic index foods may facilitate glycemic control:* multigrain bread, pumpernickel bread, whole oats, legumes, apple, lentils, chickpeas, mango, yams, brown rice Fat Eat healthful fats: low-mercury/low-contaminant-containing nuts, avocado, certain plant oils, fish Limit saturated fats (butter, fatty red meats, tropical plant oils, fast foods) and trans fats Use no- or low-fat dairy products Protein Consume protein from foods low in saturated fats (fish, egg whites, beans) Avoid or limit processed meats Micronutrients Routine supplementation not necessary except for patients at risk of insufficiency or deficiency Chromium; vanadium; magnesium; vitamins A, C, and E; and CoQ10 not recommended for glycemic control *Insufficient evidence to support a formal recommendation to educate patients that sugars have both positive and negative health effects 5 Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. AACE Medical Nutritional Therapy Recommendations • Consistency in day-to-day carbohydrate intake • Adjusting insulin doses to match carbohydrate intake (eg, use of carbohydrate counting) • Limitation of sucrose-containing or highglycemic index foods • Adequate protein intake • “Heart-healthy” diets • Weight management • Exercise • Increased glucose monitoring 6 Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. AACE Physical Activity Recommendations • ≥150 minutes per week of moderate-intensity exercise – Flexibility and strength training – Aerobic exercise (eg, brisk walking) • Start slowly and build up gradually • Evaluate for contraindications and/or limitations to increased physical activity before patient begins or intensifies exercise program • Develop exercise recommendations according to individual goals and limitations 7 Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. Glycemic Management in Type 2 Diabetes Antihyperglycemic Therapy 8 Noninsulin Agents Available for Treatment of Type 2 Diabetes Class Primary Mechanism of Action Agent Acarbose Miglitol Available as Precose or generic Glyset -Glucosidase inhibitors Delay carbohydrate absorption from intestine Decrease glucagon secretion Slow gastric emptying Increase satiety Pramlintide Symlin Biguanide Decrease HGP Increase glucose uptake in muscle Metformin Glucophage or generic Bile acid sequestrant Decrease HGP? Increase incretin levels? Colesevelam WelChol Increase glucose-dependent insulin secretion Decrease glucagon secretion Alogliptin Linagliptin Saxagliptin Sitagliptin Nesina Tradjenta Onglyza Januvia Activates dopaminergic receptors Bromocriptine Cycloset Amylin analogue DPP-4 inhibitors Dopamine-2 agonist 9 HGP, hepatic glucose production. Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Noninsulin Agents Available for Treatment of Type 2 Diabetes Class Primary Mechanism of Action Glinides Increase insulin secretion Increase glucose-dependent insulin secretion Decrease glucagon secretion Slow gastric emptying Increase satiety GLP-1 receptor agonists Sulfonylureas Thiazolidinediones Increase insulin secretion Increase glucose uptake in muscle and fat Decrease HGP Agent Nateglinide Repaglinide Available as Starlix or generic Prandin Exenatide Byetta Exenatide XR Bydureon Liraglutide Victoza Glimepiride Glipizide Amaryl or generic Glucotrol or generic Glyburide Diaeta, Glynase, Micronase, or generic Pioglitazone Actos Rosiglitazone* Avandia *Use restricted due to increased risk of myocardial infarction (MI) 10 HGP, hepatic glucose production. Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Insulins Available for the Treatment of Type 2 Diabetes Class Primary Mechanism of Action Basal Prandial Premixed Increase glucose uptake Decrease HGP Agent Detemir Glargine Available as Levemir Lantus Neutral protamine Hagedorn (NPH) Generic Aspart Glulisine Lispro Regular human Biphasic aspart Biphasic lispro NovoLog Apidra Humalog Humulin, generic NovoLog Mix Humalog Mix 11 Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Pharmacokinetics of Insulin Agent Onset (h) Peak (h) Duration (h) Considerations NPH 2-4 4-10 10-16 Glargine ~1-4 No pronounced peak* Up to 24 hours† Less nocturnal hypoglycemia compared to NPH ~0.5-1 ~2-3 Up to 8 Basal Detemir Greater risk of nocturnal hypoglycemia compared to insulin analogues Prandial Regular Aspart <0.5 ~0.5-2.5 Glulisine Lispro 12 ~3-5 Must be injected 30-45 min before a meal Injection with or after a meal could increase risk for hypoglycemia Can be injected 0-15 min before a meal Less risk of postprandial hypoglycemia compared to regular insulin * Exhibits a peak at higher dosages. † Dose-dependent. Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Combination Agents Available for the Treatment of Type 2 Diabetes Class Metformin + DPP-4 inhibitor Metformin + glinide Metformin + sulfonylurea Metformin + thiazolidinedione Thiazolidinedione + DPP-4 inhibitor Thiazolidinedione + sulfonylurea Added Agent Available as Alogliptin Kazano Linagliptin Jentadueto Sitagliptin Janumet Repaglinide Prandimet Glipizide Metaglip and generic Glyburide Glucovance and generic Pioglitazone ACTOplus Met Rosiglitazone* Avandamet Pioglitazone + alogliptin Oseni Pioglitazone Duetact Rosiglitazone* Avandaryl *Use restricted due to increased risk of myocardial infarction (MI) 13 First Principles of the AACE/ACE T2DM Algorithm • • • • Avoid hypoglycemia Avoid weight gain Consider all medication options Recognize that acquisition cost is not the total cost of a drug • Stratify therapy selection by A1C • Recognize that postprandial glucose is an important target 14 Rodbard HW, et al. Endocr Pract. 2009;15:540-559 Secondary Principles of AACE/ACE T2DM Algorithm • Adherence is improved by – Ease of use – Minimal side effects • Improved -cell performance over a longer period is possible • Multiple combinations are required 15 Rodbard HW, et al. Endocr Pract. 2009;15:540-559 Overview of AACE/ACE T2DM Algorithm • Stratify treatment based on initial A1C level • Initial monotherapy for A1C 6.5% to 7.5% • Initial dual therapy for A1C 7.6% to 9.0% • Initial triple therapy or insulin for A1C >9.0% • Monitor A1C carefully and intensify therapy at 2- to 3month intervals if A1C goal not achieved – Monotherapy → dual therapy – Dual therapy → triple therapy or insulin ± oral agents • Combine agents with different mechanisms of action 16 Rodbard HW, et al. Endocr Pract. 2009;15:540-559 Benefits are classified according to major effects on fasting glucose, postprandial glucose, and nonalcoholic fatty liver disease (NAFLD). Eight broad categories of risks are summarized. The intensity of the background shading of the cells reflects relative importance of the benefit or risk.* 17 * The abbreviations used here correspond to those used on the algorithm (Fig. 1). Available at www.aace.com/pub ** The term ‘glinide’ includes both repaglinide and nateglinide. © AACE December 2009 Update. May not be reproduced in any form without express written permission from AACE A1C 6.5 – 7.5%** A1C 7.6 – 9.0% A1C > 9.0% Under Treatment Drug Naive Symptoms Monotherapy MET † DPP4 1 GLP-1 Dual Therapy 8 TZD 2 AGI 3 2 - 3 Mos.*** MET + GLP-1 or DPP4 1 TZD 2 + Glinide or SU 5 TZD 2 - 3 Mos. INSULIN ± Other Agent(s) 6 GLP-1 or DPP4 1 MET GLP-1 or DPP4 1 + TZD 2 Colesevelam MET + MET *** + GLP-1 or DPP4 1 + SU 7 TZD 2 Triple Therapy MET + GLP-1 or DPP4 1 18 2 - 3 Mos. TZD 2 + Glinide or SU 4,7 2 - 3 Mos. INSULIN ± Other Agent(s) 6 TZD ± SU 7 2 GLP-1 or DPP4 1 INSULIN ± Other Agent(s) 6 ± TZD 2 * May not be appropriate for all patients ** For patients with diabetes and A1C < 6.5%, pharmacologic Rx may be considered *** If A1C goal not achieved safely † Preferred initial agent AGI 3 2 - 3 Mos. + *** Triple Therapy 9 GLP-1 or DPP4 1 + GLP-1 or DPP4 1 or TZD 2 SU or Glinide 4,5 Dual Therapy MET No Symptoms *** INSULIN ± Other Agent(s) 6 *** AACE/ACE Algorithm for Glycemic Control Committee Cochairpersons: Helena W. Rodbard, MD, FACP, MACE Paul S. Jellinger, MD, MACE Zachary T. Bloomgarden, MD, FACE Jaime A. Davidson, MD, FACP, MACE Daniel Einhorn, MD, FACP, FACE Alan J. Garber, MD, PhD, FACE James R. Gavin III, MD, PhD George Grunberger, MD, FACP, FACE Yehuda Handelsman, MD, FACP, FACE Edward S. Horton, MD, FACE Harold Lebovitz, MD, FACE Philip Levy, MD, MACE Etie S. Moghissi, MD, FACP, FACE Stanley S. Schwartz, MD, FACE 1 DPP4 if PPG and FPG or GLP-1 if PPG 2 TZD if metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD) 3 AGI if PPG 4 Glinide if PPG or SU if FPG 5 Low-dose secretagogue recommended 6 a) Discontinue insulin secretagogue with multidose insulin b) Can use pramlintide with prandial insulin 7 Decrease secretagogue by 50% when added to GLP-1 or DPP-4 8 If A1C < 8.5%, combination Rx with agents that cause hypoglycemia should be used with caution 9 If A1C > 8.5%, in patients on Dual Therapy, insulin should be considered Available at www.aace.com/pub © AACE December 2009 Update. May not be reproduced in any form without express written permission from AACE LIFESTYLE MODIFICATION AACE/ACE DIABETES ALGORITHM FOR GLYCEMIC CONTROL A1C 6.5 – 7.5%** Monotherapy MET † DPP4 1 GLP-1 TZD 2 AGI 3 2 - 3 Mos.*** Dual Therapy GLP-1 or DPP4 1 MET TZD 2 + Glinide or SU 5 TZD + MET + GLP-1 or DPP4 1 Colesevelam *** If A1C goal not achieved safely † Preferred initial agent 1 DPP4 if PPG and FPG or GLP-1 if PPG 2 TZD if metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD) TZD 2 3 AGI if PPG 4 Glinide if PPG or SU if FPG Glinide or SU 4,7 5 Low-dose secretagogue recommended 2 - 3 Mos.*** 6 a) Discontinue insulin secretagogue with multidose insulin b) Can use pramlintide with prandial insulin 7 Decrease secretagogue by 50% when added to GLP-1 or DPP-4 AGI 3 2 - 3 Mos. *** Triple Therapy MET + GLP-1 or DPP4 1 + INSULIN ± Other Agent(s) 6 19 Available at www.aace.com/pub © AACE December 2009 Update. May not be reproduced in any form without express written permission from AACE LIFESTYLE MODIFICATION A1C 7.6 – 9.0% AACE/ACE DIABETES ALGORITHM FOR GLYCEMIC CONTROL Dual Therapy 8 MET + GLP-1 or DPP4 1 or TZD 2 SU or Glinide 4,5 2 - 3 Mos.*** Triple Therapy 9 GLP-1 or DPP4 1 MET + GLP-1 or DPP4 1 + TZD 2 + SU 7 *** If A1C goal not achieved safely † Preferred initial agent 1 DPP4 if PPG and FPG or GLP-1 if PPG 2 TZD if metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD) 4 Glinide if PPG or SU if FPG 5 Low-dose secretagogue recommended 6 a) Discontinue insulin secretagogue with multidose insulin b) Can use pramlintide with prandial insulin 7 Decrease secretagogue by 50% when added to GLP-1 or DPP-4 8 If A1C < 8.5%, combination Rx with agents that cause hypoglycemia should be used with caution 9 If A1C > 8.5%, in patients on Dual Therapy, insulin should be considered TZD 2 2 - 3 Mos. INSULIN ± Other Agent(s) *** 6 20 Available at www.aace.com/pub © AACE December 2009 Update. May not be reproduced in any form without express written permission from AACE LIFESTYLE MODIFICATION Drug Naive Symptoms INSULIN ± Other Agent(s) AACE/ACE DIABETES ALGORITHM FOR GLYCEMIC CONTROL A1C > 9.0% Under Treatment No Symptoms GLP-1 or DPP4 1 MET + ± SU 7 TZD 2 6 GLP-1 or DPP4 1 ± TZD 2 INSULIN ± Other Agent(s) 6 1 DPP4 if PPG and FPG or GLP-1 if PPG 2 TZD if metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD) 6 a) Discontinue insulin secretagogue with multidose insulin b) Can use pramlintide with prandial insulin 7 Decrease secretagogue by 50% when added to GLP-1 or DPP-4 21 Available at www.aace.com/pub © AACE December 2009 Update. May not be reproduced in any form without express written permission from AACE Basal Insulin Therapy in T2DM: AACE/ACE Recommendations • Initiate insulin treatment by adding a long-acting basal formulation to existing noninsulin agents Basal insulin analogues (detemir, degludec,* or glargine) are strongly preferred over human NPH insulin Relatively peakless time-action curves • • • Greater dayto-day consistency Lower risk of hypoglycemia Start with 10 U or 0.1-0.2 U/kg per day at bedtime Slowly titrate by 1-3 U every 2-3 days until FPG reaches the desired target (<100 mg/dL for most patients) Decrease dosage if FPG declines below a threshold specified for individual patient 22 *Under FDA review as of October 2012. Rodbard HW, et al. Endocr Pract. 2009;15:540-559. Prandial Insulin Therapy in T2DM: AACE/ACE Recommendations • Add prandial insulin when A1C levels remain high despite optimal control of FPG with basal insulin ± noninsulin agents • Basal-bolus insulin therapy is flexible and is recommended for intensive insulin therapy • Premixed insulin analogues – Consider for patients with adherence problems – Lack dosage flexibility and may increase risk of hypoglycemia Rapid-acting insulin analogues are preferred over regular human insulin Faster onset of action Faster offset of action Lower risk of hypoglycemia 23 Rodbard HW, et al. Endocr Pract. 2009;15:540-559. Early Insulin Use in Type 2 Diabetes Offers No Benefits Over Standard Approaches Outcome Reduction With an Initial Glargine Intervention CV risk factors + prediabetes or T2DM (N=12,537) 24 ORIGIN Trial Investigators. N Engl J Med. 2012;367:319-328. AACE/ACE T2DM Algorithm: Special Considerations and Caveats • Metformin is the preferred initial agent for most patients • DPP-4 inhibitors are preferred if both PPG and FPG are elevated • GLP-1 agonists are preferred if the principal problem is elevated PPG • TZDs can be used to treat patients with metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD) • AGIs are useful for treatment of elevated PPG • Glinides can be useful for treatment of elevated PPG • SUs may be useful if major problem is elevated FPG • Colesevelam may be useful for patients near A1C goal but needing additional LDL-C control 25 Rodbard HW, et al. Endocr Pract. 2009;15:540-559. AACE/ACE T2DM Algorithm: Special Considerations and Caveats • A1C goal ≤6.5% may not be • If A1C is <8.5%, combination appropriate for all patients therapy with agents that cause hypoglycemia should be used • For patients with diabetes and with caution A1C <6.5%, pharmacologic – Decrease dose of secretagogue therapy may still be considered by 50% when added to GLP-1 • If A1C goal is not achieved, or DPP-4 intensify therapy (if it can be • If A1C ≥8.5% in patients on dual done safely) therapy, consider use of insulin – Discontinue insulin secretagogue with multi-dose insulin – Consider use of pramlintide with prandial insulin 26 Rodbard HW, et al. Endocr Pract. 2009;15:540-559. ADA/EASD T2DM Treatment Algorithm 27 Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. ADA/EASD T2DM Treatment Algorithm: Sequential Insulin Strategies 28 Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Common Principles in AACE/ACE and ADA/EASD T2DM Treatment Algorithms • Individualize glycemic goals based on patient characteristics • Promptly intensify antihyperglycemic therapy to maintain blood glucose at individual targets – Combination therapy necessary for most patients – Base choice of agent(s) on individual patient medical history, behaviors and risk factors, ethno-cultural background, and environment • Insulin eventually necessary for many patients • SMBG vital for day-to-day management of blood sugar – All patients using insulin – Many patients not using insulin 29 Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Rodbard HW, et al. Endocr Pract. 2009;15:540-559. Pipeline Classes and Agents (2012) Class Phase of Development Dual peroxisome proliferator activated receptor - (PPAR-) agonist Phase 3 Short-acting GLP-1 receptor agonist Long-acting GLP-1 receptor agonists Phase 3 Insulin Phase 3 Description Lixisenatide Improve insulin sensitivity in the periphery as well as lipid profiles Approved agents may reduce both cardiovascular risks and potential for diabetes complications Human-derived molecule with effects similar to exenatide Albiglutide Taspoglutide Effects probably similar to currently available GLP-1 receptor agonists Longer duration of action will permit longer intervals between injections Aleglitazar Degludec DegludecPlus Ultra-long-acting basal insulin (half-life ~25 hours) with low within-subject variability and potential for reduced incidence of hypoglycemia Premixed insulin containing degludec plus aspart, providing both fasting and postprandial glucose control Generically available anti-inflammatory medication currently approved for treatment of arthritis; inhibits activity of NF-B, an inflammatory factor Salicylates Phase 3 Salsalate Sodium-dependent glucose cotransporter 2 (SGLT-2) inhibitors Phase 3 Canagliflozin Dapagliflozin Empagliflozin Tofogliflozin Act in the kidney Reduce hyperglycemia by inhibiting glucose reabsorption into the bloodstream from the renal filtrate, increasing urinary excretion of glucose INCB13739 RG4929 Inhibit 11HSD-1 mediated conversion of low-activity cortisone to cortisol, which is primarily produced in the liver and adipose tissue May lessen stress-induced obesity, improve insulin sensitivity, enhance insulin-secretory responsiveness, and improve glucose tolerance in patients with metabolic syndrome and/or type 2 diabetes 11-Hydroxysteroid dehydrogenase type 1 (11HSD-1) inhibitors Phase 2 30 Agents Bakris GL, et al. Kidney Int. 2009;75:1272-1277; Calado J, et al. Kidney Int Suppl. 2011:S7-S13; Garber AJ. Expert Opin Investig Drugs. 2012;21:45-57; Goldfine AB, et al. Ann Intern Med. 2010;152:346-357; King A. J Fam Pract. 2012;61:S28-S31; Tahrani AA, et al. Lancet. 2011;378:182-197; Tahrani AA, et al. Lancet. 2012;379:1465-1467. Glycemic Management in Type 2 Diabetes Technology for Type 2 Diabetes Management 31 SMBG in Type 2 Diabetes: AACE/ACE Recommendations Noninsulin Users • Introduce at diagnosis • Personalize frequency of testing • Use SMBG results to inform decisions about whether to target FPG or PPG for any individual patient Testing positively affects glycemia in T2DM when the results are used to: • Modify behavior • Modify pharmacologic treatment Insulin Users • All patients using insulin should test glucose – ≥2 times daily – Before any injection of insulin • More frequent SMBG (after meals or in the middle of the night) may be required – Frequent hypoglycemia – Not at A1C target 32 SMBG, self-monitoring of blood glucose. Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. SMBG in Noninsulin Using Patients With T2DM Adjusted Mean A1C (%) 9.0 Active control group (n=227) Structured testing group (n=256) 8.8 8.6 8.4 8.2 8.0 -0.3% 7.8 (P=0.04) 7.6 7.4 7.2 Baseline M1 M3 M6 M9 M12 ACG 8.9% (0.08) 8.7% (0.1) 8.2% (0.1) 7.9% (0.1) 8.0% (0.1) 8.0% (0.1) STG 8.9% (0.07) 8.5% (0.09) 7.9% (0.09) 7.9% (0.09) 7.6% (0.09) 7.7% (0.09) 33 ACG, active cotnrol group; STG, structured testing group. Polonsky WH, et al. Diabetes Care. 2011;34:262-267. CSII in Type 2 Diabetes: Patient Candidates • Absolutely insulin-deficient • Take 4 or more insulin injections a day • Assess blood glucose levels 4 or more times daily • Motivated to achieve tighter glucose control • Mastery of carbohydrate counting, insulin correction, and adjustment formulas • Ability to troubleshoot problems related to pump operation and plasma glucose levels • Stable life situation • Frequent contact with members of their healthcare team, in particular their pumpsupervising physician 34 CSII, continuous subcutaneous insulin infusion. Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. Glycemic Management in Type 2 Diabetes Surgical Intervention 35 Surgical Intervention in Type 2 Diabetes 3.5 3.0 2.5 2.0 1.5 1.0 0.5 0.0 Baseline P<0.001 P<0.001 3 6 9 12 FPG (mg/dL) 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 Baseline Sleeve gastrectomy Roux-en-Y gastric bypass 20 0 -20 -40 -60 -80 -100 -120 -140 -160 Baseline P=0.02 P<0.001 3 6 9 12 0 P<0.001 P<0.001 3 6 Months 9 12 BMI (kg/m2) Average no. diabetes medications A1C (%) Intensive medical therapy -2 -4 -6 P<0.001 -8 -10 -12 Baseline P<0.001 3 6 9 12 Months 36 Schauer PR, et al. N Engl J Med. 2012;366:1567-1576. Glycemic Management in Type 2 Diabetes Safety Concerns: Hypoglycemia 37 Type 2 Diabetes Pathophysiology: Origins of Hypoglycemia Defect β-cells Increased insulin availability due to use of secretagogues or exogenous insulin Liver Suppressed hepatic glucose production due to impaired counterregulatory response Skeletal muscle Increased glucose uptake due to exercise α-cells Suppressed glucagon due to impaired counter-regulatory response Brain Hypoglycemia unawareness 38 Cryer PE. Am J Physiol. 1993; 264(2 Pt 1):E149-E155. Hypoglycemia: Risk Factors Patient Characteristics Behavioral and Treatment Factors • Older age • Female gender • African American ethnicity • Longer duration of diabetes • Neuropathy • Renal impairment • Previous hypoglycemia • Missed meals • Elevated A1C • Insulin or sulfonylurea therapy 39 Miller ME, et al. BMJ. 2010 Jan 8;340:b5444. doi: 10.1136/bmj.b5444. Symptoms and Signs with Progressive Hypoglycemia Blood Glucose (mg/dL) 100 90 80 70 60 50 40 30 20 10 Decreased insulin secretion Increased glucagon, epinephrine, ACTH, cortisol, and growth hormone Palpitation, sweating Decreased cognition, hunger Aberrant behavior Seizures, coma Neuronal cell death 0 Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Hypoglycemia: Clinical Consequences Acute • Symptoms (sweating, irritability, confusion) • Accidents • Falls Long-term • Recurrent hypoglycemia and hypoglycemia unawareness • Refractory diabetes • Dementia (elderly) • CV events – Cardiac autonomic neuropathy – Cardiac ischemia – Fatal arrhythmia – Angina 41 Cryer PE, et al. Diabetes Care. 2003;26:1902-1912. ADA. Diabetes Care. 2013;36(suppl 1):S11-S66. Zammit NN, et al. Diabetes Care. 2005;28:2948-2961. Treatment of Hypoglycemia: AACE/ACE Recommendations Blood Glucose Level ~50-60 <50 Classification Typical Signs and Symptoms Treatment Mild hypoglycemia Moderate hypoglycemia Severe hypoglycemia Neurogenic: palpitations, tremor, hunger, sweating, anxiety, paresthesia Neuroglycopenic: behavioral changes, emotional lability, difficulty thinking, confusion Severe confusion, unconsciousness, seizure, coma, death Requires help from another individual Consume glucose-containing foods (fruit juice, soft drink, crackers, milk, glucose tablets); avoid foods also containing fat Repeat glucose intake if SMBG result remains low after 15 minutes Consume meal or snack after SMBG has returned to normal to avoid recurrence Glucagon injection, delivered by family member or other close associate Victim should be taken to hospital for evaluation and treatment after any severe episode 42 Cryer PE, et al. Diabetes Care. 2003;26:1902-1912. Elements of Hypoglycemia Prevention Set appropriate glycemic targets for individual patients More stringent goals: young, newly diagnosed, no comorbidities, no micro- or macrovascular disease, strong and effective self-care skills Less stringent goals: older, limited life expectancy, history of hypoglycemia, longer disease duration, established comorbidities, established vascular disease, limited selfcare skills Signs and symptoms of hypoglycemia Dietary education for improved glycemic control and appreciation of triggers for hypoglycemia Avoiding missed or delayed meals Appropriate self-treatment Understanding of hypoglycemia unawareness Importance of reporting hypoglycemia Use self-monitoring of blood glucose Patient education: technique and action Observation of patient’s procedure and reaction Patient access to providers for purposes of reporting results and for providing guidance Provider reaction to results increases effectiveness of SMBG Hold a high index of suspicion for hypoglycemia Understand patients may not report “typical” symptoms When hypoglycemia is suspected, adjust therapy Consider use of continuous glucose monitoring to detect unrecognized hypoglycemia Choose appropriate therapy Use agents with a low risk of hypoglycemia Be aware of additive effects of combination therapies on hypoglycemia risk Recognize that long-term costs of hypoglycemia may offset the cost of using older, less physiologic medications Educate patients 43 Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Hypoglycemia Risk With Antihyperglycemic Agents Added to Metformin Initial Treatment Additional Treatment DPP-4 inhibitors Less Hypoglycemia GLP-1 receptor agonists TZDs Metformin More Hypoglycemia Sulfonylureas Insulin (basal, basal-plus, premixed) Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Frequency of Severe Hypoglycemia With Antihyperglycemic Agents Percentage of Patients Treated in 1 Year 6% Mixtures, Rapid-acting, Basal-bolus 5% Insulin 4% Basal 3% 2% 1% 0 Sulfonylureas Meglinitides DPP-4 inhibitors, GLP-1 receptor agonists, Metformin, TZDs Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Relative Rates of Severe Hypoglycemia with Insulin Increasing rates of hypoglycemia Most frequent Prandial and premixed Human insulin Analogue insulins Premixed (70/30, 75/25) More frequent Basal + Basal plus 2-3 prandial Basal plus one prandial Basal only NPH Basal analogues (glargine, detemir) Pipeline basal analogues (degludec, pegylated lispro) Less frequent Moghissi E, et al. Endocr Pract. 2013;Feb 20:1-33. [Epub ahead of print]. Glycemic Management in Type 2 Diabetes Safety Concerns: Weight 47 Antidiabetic Agents and Weight Weight Gain Weight Neutral or Weight Loss Glinides Insulin Sulfonylureas Thiazolidinediones -Glucosidase inhibitors Bromocriptine Colesevelam DPP-4 inhibitors GLP-1 receptor agonists Metformin Pramlintide • Risk of additional weight gain must be balanced against the benefits of the agent – Sulfonylureas may negate weight loss benefits of GLP-1 receptor agonists or metformin – Insulin should not be withheld because of the risk of weight gain 48 Inzucchi SE, et al. Diabetes Care. 2012;35:1364-1379. Rodbard HW, et al. Endocr Pract. 2009;15:540-559. Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. Glycemic Management in Type 2 Diabetes Safety Concerns: Cancer Risk 49 Insulin and Cancer Risk Study Hazard Ratio (95% CI) Outcome Reduction With an Initial Glargine Intervention (ORIGIN) N=12,537; prospective RCT Median follow-up: 6.2 years Any cancer: 1.00 (0.88-1.13); P=0.97 Death from cancer: 0.94 (0.77-1.15); P=0.52 Northern European Database Study N=447,821; observational Mean follow-up: Glargine users: 3.1 years Other insulin users: 3.5 years Breast cancer (women): 1.12 (0.99-1.27) Prostate cancer (men): 1.11 (1.00-1.24) Colorectal cancer (men and women): 0.86 (0.76-0.98) Kaiser-Permanente Collaboration N=115,000; observational Median follow-up: Glargine users: 1.2 years NPH users: 1.4 years Breast cancer (women): 1.0 (0.9-1.3) Prostate cancer (men): 0.7 (0.6-0.9) Colorectal cancer (men and women): 1.00 (0.8-1.2) All cancers (men and women): 0.9 (0.9-1.0) MedAssurant Database Study N=52,453; observational Mean follow-up: Glargine users: 1.2 years NPH users: 1.1 years No increased risk for breast cancer 50 ORIGIN Trial Investigators. N Engl J Med. 2012;367:319-328. Kirkman MS, et al. Presented at the American Diabetes Association 72nd Scientific Sessions. June 11, 2012. Session CT-SY13. Philadelphia, PA. Glycemic Management in Type 2 Diabetes Special Populations and Situations 51 Management Considerations for Elderly Patients with Diabetes Increased risk of and from falling • Impaired vision • Reduced strength and stamina • Sensitivity to medication side effects • Frailty • Susceptibility to hypoglycemia Hypoglycemia unawareness and recurrent hypoglycemia • Impaired counterregulatory mechanisms Other complicating factors • Diminished kidney function • Urinary incontinence • Status of social support and/or caregiver • Drug-drug interactions Consider risks before prescribing: • Sulfonylureas and glinides (hypoglycemia risk) • Thiazolidinediones (fracture risk) • Metformin (risk of lactic acidosis with decreased kidney function) Impaired capacity, understanding, and/or motivation for proper self-care • Cognitive decline and dementia • Depression • Impaired vision Consider when establishing treatment goals • Patient overall health and well-being • Self-care capacities • Social/family support 52 Bourdel Marchasson I, et al. J Nutr Health Aging. 2009;13:685-691. Handelsman Y, et al. Endocr Pract. 2011;17(suppl 2):1-53. Schwartz AV, et al. Diabetes Care. 2008;31:391-396. Zammitt NN, Frier BM. Diabetes Care. 2005;28:2948-2961. Risk Considerations for Religious/Cultural Fasting Main Risks of Fasting • • • • Risk Category Low Moderate High Very high 53 Hypoglycemia Hyperglycemia Diabetic ketoacidosis Dehydration and thrombosis Features Glycemia well-controlled with antihyperglycemic agent that does not cause hypoglycemia (eg, metformin, thiazolidinedione, DPP-4 inhibitor, GLP-1 receptor agonist) Otherwise healthy Glycemia well-controlled with glinides Moderate hyperglycemia (A1C 7.5-9.0%), renal insufficiency, cardiovascular complications, and/or other comorbid conditions Living alone, especially if taking sulfonylureas, insulin, or drugs that affect mentation Elderly, especially with poor health History of recurrent hypoglycemia, hypoglycemia unawareness, or episode of severe hypoglycemia within 3 months prior to Ramadan Poor glycemic control Ketoacidosis or hyperosmotic hyperglycemic coma within 3 months prior to Ramadan Acute illness or chronic dialysis Intense physical labor Pregnancy Al-Arouj M, et al. Diabetes Care. 2005;28:2305-2311. Glycemic Management During Religious/Cultural Fasting • Frequent glucose monitoring—break fast immediately if patient has: – Hypoglycemia • SMBG <70 mg/dL while taking insulin or sulfonylureas • SMBG <60 mg/dL while on other therapies – Hyperglycemia: >300 mg/dL • Healthful eating before and after each fasting period – Complex carbohydrates prior to fast – Avoid ingesting high-carbohydrate, high-fat foods when breaking fast • Avoid excessive physical activity but maintain normal exercise routines • Avoid fasting while ill 54 Al-Arouj M, et al. Diabetes Care. 2005;28:2305-2311.