Organic Chemistry
advertisement

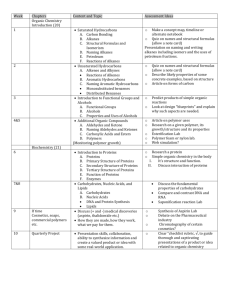
Erin Nolan Nature of Science - Investigating in science Develop and carry out investigations that extend their scientific knowledge, including developing their understanding of the relationship between investigations and scientific theories and models - Communicating in science Use accepted science knowledge, vocabulary, symbols, and conventions when evaluating accounts of the nature world and consider wider implications of the methods of communication and/or representation employed. ‣ Material World - Properties and changes of matter Investigate and measure the chemical and physical properties of a range of groups of substances, for example, acids and bases, oxidants and reductants, and selected organic and inorganic compounds - The structure of matter Relate properties of matter to structure and bonding. Develop an understanding of and use the fundamental concepts of chemistry (for example equilibrium and thermochemical principles) to interpret observations. Alkanes Alkenes and Alkynes Polymers Alcohols Carboxylic Acids Esters Subtopic Activity Naming Alkanes Fill in the blanks table, which will help scaffold the answers out of the students. Also, molymod kits to help the students understand how the naming system works. In addition, students will need to name branched alkanes which could be done with a fill-in table as well. Structural Isomers Molymod kits to make up isomers, so students can . A table to fill in with just molecular formulae and students can work out the structural isomers. Haloalkanes Not only is their naming with haloalkanes but also classification which the students would not have come across before. Another fill in the blanks table will help scaffold them again as they will come across this type of classification system again with alcohols. Physical Properties and Reactions Experiment testing and observing how different alkanes burn, their states at room temperature, solubility in water, conduction of electricity. Sub-topic Activity Naming alkenes and alkynes Naming an alkene and alkyne is different to how you name an alkane. However, parts of an alkane name is used as basis for naming these two other compounds. So a refresher of alkane names will help and then some fill-in tables introducing branched alkenes and alkynes to further expand on what they have previously covered with branched alkanes. Geometrical isomers Molymod kits. Students can fill in a table that has a list of molecular formulae and also make up models of these molecules so that they can get a 3D appreciation of the isomers. Reactions Experiment. Students will do an experiment using an alkane and an alkene and explain the differences they observe such as why cyclohexene makes bromine water become colourless without the presence of a catalyst whereas cyclohexane needs bright light. Polymers Molymod kits, microscope photos of polymers and bringing in everyday items made out of polymers such as polypropylene, nylon. Sub-topic Activity Naming and classification Review of alkene naming procedure as this one is very similar and review of classification of haloalkanes as it is the same method for alcohols. A fill-in table could be used as well. Preparation Chalk and Talk, informing students how industrial methanol and ethanol are produced. Properties and Reactions Students will get to examine different alcohols and how differing molar masses affect their physical properties. Also conduct an experiment to see how they combust and their solubility in water which can be related to the bonding in different alcohols. Another reaction of alcohols would be with acidified potassium permanganate and acidified potassium dichromate. Sub-topic Activity Naming Revision of alkane names as they form for the basis for carboxylic acid names. Also, revision of branched chain alkanes as it is the same naming method for carboxylic acids. Properties and Reactions Experiment. Have a range of carboxylic acids for students to describe their physical properties such as smell, states at room temperature. Also react them with water and universal indicator and describe and name the end product. Sub-topic Activity Naming and formation Experiment. React a carboxylic acid with an alcohol and get students to describe the end product and the smell. Then go over the naming and provide a table for them to work through. Hydrolysis Experiment. This time react the ester with water or NaOH which will cause it to split. The students will then get to write reactions for the reverse of what they had done before. Fats and Oils Get students to bring some household examples of fats (eg lard) and oils and get them to describe the states at room temperatures. Soap Experiment. Get students to make their own soap. Students should be able to describe how soap is formed. Organic chemistry is a difficult subject to make relevant to students as most things that you describe to them they can’t actually see and observe themselves, such as the addition of bromine to an alkene. But we do know the reaction has occurred as bromine water becomes colourless, however you cannot observe the alkene double bond breaking and a bromine atom being added to the resulting alkane. But what could be done in this subject is to bring in everyday household substances that are made up of organic chemical molecules such as polymers and alkanes so that it may make it more relevant to the students. For example for polymers Polypropylene and nylon could be brought in and for alkanes candles. Hopefully the students will then gain an appreciation of what some of their items of clothing that they wear or what is at home may contain. Also, vinegar could be brought in an described in terms of what organic molecules it is made of. Students would be dealt with on a case by case basis. For example if a student was hard of sight, then a student or a teacher aide paired up with them to help them with experimental work and also enlarged printed copies may have to be provided. ESOL is another situation that will be dealt with case by case basis. A student maybe placed in a group of New Zealand students to aide their understanding and get a grip on the terminology used in chemistry. On the other hand being placed students of the same nationality may also aide their learning.