2 - Functional Groups Notes Handout
advertisement

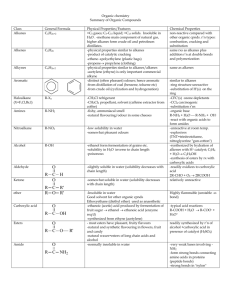
15/03/2016 Science 30 Unit B – Functional Groups Name: __________ Date: __________ Recall: organic chemicals are mostly made up of ______________ and ____________ atoms bonded together in straight lines...however, we can also add other atoms to these chains or rings to spice things up a bit. These added atoms are called functional groups. 1. Alcohols- all alcohols contain the hydroxyl functional group, OH Naming Alcohols: the naming is no different from alkanes, except... Step 1: drop the e from the ane ending. Step 2: add -ol. ex) CH3OH - methane – _________________ ex) CH3CH2OH - ethane – _______________ Step 3: of the hydroxyl group is not on the last carbon, you need to say which carbon it comes after. ex) Write name of each alcohol. a) b) ex) Write condensed structural formulas for each alcohol. a) butanol www.ldindustries.ca b) hexan-2-ol 15/03/2016 2. Carboxylic Acids - all carboxylic acids contain the carboxyl functional group, COOH - this is simply a double bonded oxygen and a hydroxyl Naming Carboxylic Acids: the naming is no different from alkanes, except... Step 1: drop the e from the ane ending. Step 2: add -oic acid. Fun Fact: Some common carboxylic acids include: - ethanoic acid (acetic acid) - vinegar - butanoic acid - butter - hexanoic acid - goat fat - tetradecanoic acid - nutmeg - octadecanoic acid - chocolate ex) CHOOH - methane – methanoic acid (HCOOH) ex) CH3CH2OOH - ethane - ethanoic acid ex) Name each compound. a) 3. Esters - esters are formed when an alcohol reacts with a carboxylic acid. Naming Esters: Step 1: name the chain attached to the single bonded oxygen (this came from the alcohol). Step 2: name the chain attached to the double bonded oxygen (this came from the carboxylic acid). Step 3: stick the names together and add "oate" on the end. ex) Name the ester. a) www.ldindustries.ca b) 15/03/2016 ex) Draw a condensed structural diagram of propyl ethanoate. Fun Fact: Esters form many of the smells of foods, such as: pineapple, pear, strawberry, apple, pine, cinnamon, etc. 4. Halogenated Hydrocarbons - all halogenated hydrocarbons contain at least one halogen. Naming Halogenated Hydrocarbons: is no different from alkanes, except... Step 1: use a prefix for the number of halogens (di, tri, tetra, etc) Step 2: write the number the halogen is attached to. ex) Name the halogenated hydrocarbon. Unfun Fact: Many halogenated hydrocarbons are extremely harmful to the environment: CFC (refrigerator coolants) deplete the ozone layer. DDT (a pesticide) biomagnifies in the food chain and causes deaths in animals. Practice: Functional Groups WS www.ldindustries.ca