Continuation of CHAPTER 11
advertisement

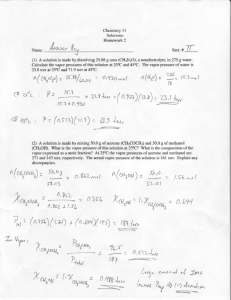
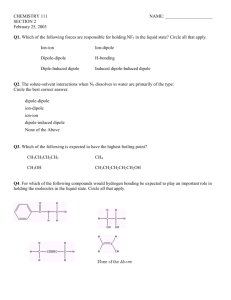
Continuation of CHAPTER 11 CHEM 1212 Vapor Pressure • Vapor pressure is the pressure exerted when the liquid and vapor are in dynamic equilibrium. • Dynamic Equilibrium - the point when as many molecules escape the surface as strike the surface. Prentice Hall © 2003 Chapter 11 What you have learned so far……. • Some compounds are volatile (evaporates easily) because… (a) the molecules are held together by weak intermolecular forces therefore it doesn’t take much E to break the bonds and so they evaporate easily. • Compounds that are volatile have lower boiling points. Chapter 11 Clausius-Clapeyron Equation • ln P = - DHvap + C RT Where: T = absolute temperature R = gas constant (8.314 J/K-mol) DH = heat of vaporization P = vapor pressure C = constant IDEAL GAS CONSTANT • R = 0.08206 liter . atm/K . Mole • OR • R = 8.314 J/K . Mole • Conversion 101.325 J = 1 L.atm Vapor Pressure and Boiling Pt. • Sample Problem • If the normal boiling point of water is 100. oC, what will be its boiling point at 735 torr? The heat of vaporization of water is 40.67 kJ/mole. Clausius-Clapeyron Equation • To determine the effect of changing temperature or vapor pressure, use the following equation: • ln [P1/P2] = - DHvap [1/T1 - 1/T2] R Clausius-Clapeyron Equation So your very first equation in Chem 1212 is: • ln [P1/P2] = DHvap [1/T2 - 1/T1] R Problem CCl4 has a vapor pressure of 213 o torr at 40 C and 836 torr at 80 oC. What is the heat of vaporization of CCl4? Extra Problem • The melting point of potassium is 63.2 oC. Molten potassium has a vapor pressure of 10.0 torr at 443 oC and a vapor pressure of 400.0 torr at 708 oC. • A.) Calculate the heat of vaporization of liquid potassium. • B.) Calculate the normal boiling point of potassium. • C.) Calculate the vapor pressure of liquid potassium at 100 oC.