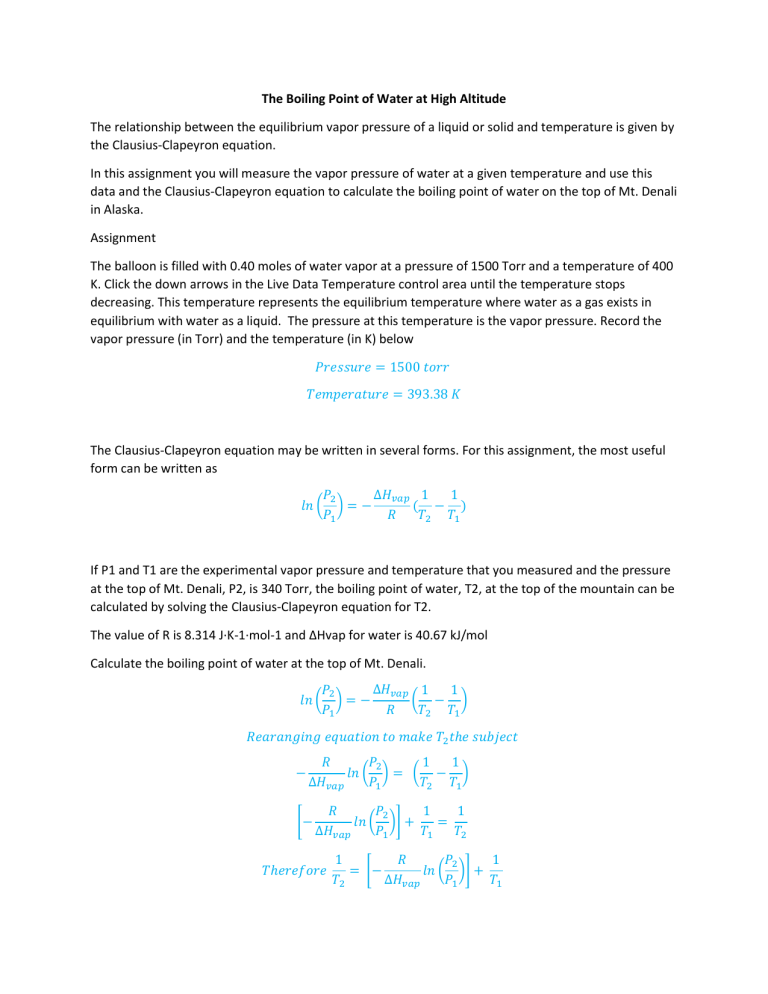
The Boiling Point of Water at High Altitude The relationship between the equilibrium vapor pressure of a liquid or solid and temperature is given by the Clausius-Clapeyron equation. In this assignment you will measure the vapor pressure of water at a given temperature and use this data and the Clausius-Clapeyron equation to calculate the boiling point of water on the top of Mt. Denali in Alaska. Assignment The balloon is filled with 0.40 moles of water vapor at a pressure of 1500 Torr and a temperature of 400 K. Click the down arrows in the Live Data Temperature control area until the temperature stops decreasing. This temperature represents the equilibrium temperature where water as a gas exists in equilibrium with water as a liquid. The pressure at this temperature is the vapor pressure. Record the vapor pressure (in Torr) and the temperature (in K) below 𝑃𝑟𝑒𝑠𝑠𝑢𝑟𝑒 = 1500 𝑡𝑜𝑟𝑟 𝑇𝑒𝑚𝑝𝑒𝑟𝑎𝑡𝑢𝑟𝑒 = 393.38 𝐾 The Clausius-Clapeyron equation may be written in several forms. For this assignment, the most useful form can be written as ∆𝐻𝑣𝑎𝑝 1 𝑃2 1 𝑙𝑛 ( ) = − ( − ) 𝑃1 𝑅 𝑇2 𝑇1 If P1 and T1 are the experimental vapor pressure and temperature that you measured and the pressure at the top of Mt. Denali, P2, is 340 Torr, the boiling point of water, T2, at the top of the mountain can be calculated by solving the Clausius-Clapeyron equation for T2. The value of R is 8.314 J·K-1·mol-1 and ΔHvap for water is 40.67 kJ/mol Calculate the boiling point of water at the top of Mt. Denali. ∆𝐻𝑣𝑎𝑝 1 𝑃2 1 𝑙𝑛 ( ) = − ( − ) 𝑃1 𝑅 𝑇2 𝑇1 𝑅𝑒𝑎𝑟𝑎𝑛𝑔𝑖𝑛𝑔 𝑒𝑞𝑢𝑎𝑡𝑖𝑜𝑛 𝑡𝑜 𝑚𝑎𝑘𝑒 𝑇2 𝑡ℎ𝑒 𝑠𝑢𝑏𝑗𝑒𝑐𝑡 − 𝑅 𝑃2 1 1 𝑙𝑛 ( ) = ( − ) ∆𝐻𝑣𝑎𝑝 𝑃1 𝑇2 𝑇1 [− 𝑅 𝑃2 1 1 𝑙𝑛 ( )] + = ∆𝐻𝑣𝑎𝑝 𝑃1 𝑇1 𝑇2 𝑇ℎ𝑒𝑟𝑒𝑓𝑜𝑟𝑒 1 𝑅 𝑃2 1 = [− 𝑙𝑛 ( )] + 𝑇2 ∆𝐻𝑣𝑎𝑝 𝑃1 𝑇1 𝑃1 = 1500 𝑇𝑜𝑟𝑟, 𝑃2 = 340 𝑇𝑜𝑟𝑟, 𝑇1 = 393.38 𝐾 𝑎𝑛𝑑 𝑇2 =? 𝑅 = 8.314 𝐽 ∙ 𝐾 −1 ∙ 𝑚𝑜𝑙 −1 ∆𝐻𝑣𝑎𝑝 = 40.67𝐾𝐽/𝑚𝑜𝑙 = 40.67 × 103 𝐽 ∙ 𝑚𝑜𝑙 −1 1 8.314 𝐽 ∙ 𝐾 −1 ∙ 𝑚𝑜𝑙 −1 340 𝑇𝑜𝑟𝑟 1 = [− × 𝑙𝑛 ( )] + 3 −1 𝑇2 40.67 × 10 𝐽 ∙ 𝑚𝑜𝑙 1500 𝑇𝑜𝑟𝑟 393.38 𝐾 1 1 = [3.034 × 10−4 ]𝐾 −1 + 𝑇2 393.38 𝐾 1 = (2.8455 × 10−3 )𝐾 −1 𝑇2 𝑇2 = 1 (2.8455 × 10−3 )𝐾 −1 𝑇2 = 351.4 𝐾 Therefore, the boiling point of water on top of Mt. Denali = 351.4 K






