Free Energy, Vapor Pressure and the Equilibrium Between a Vapor
advertisement

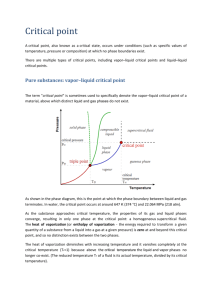
Free Energy, Vapor Pressure and the Equilibrium Between a Vapor and Condensed Phase References: Thermodynamics, G. N. Lewis and M. Randall, rev. K. Pitzer and L. Brewer, McGraw Hill, New York (1961). Chemical Thermodynamics, I. Klotz, Benjamin, New York (1964). • The Gibbs Free Energy is generally agreed to be the “weapon of choice” for describing (a) chemical reactions and (b) equilibria between phases. It is defined as: • G = H – TS = E + PV – TS (1) • Where H = Enthalpy • E = Total internal energy • T = [Absolute] Temperature • S = Entropy • Obviously dG = dE + PdV +VdP – TdS – SdT Aside • Remember that thermodynamic variables come in pairs • One is “intrinsic” (does not depend on system size) • One is “extrinsic” (depends on system size) • Examples: P and V, T and S… • Also G and N, the number of moles of stuff in the system. • Hence G is the appropriate variable when material is moving between phases From the First Law of Thermodynamics • dE = TdS – PdV since dS = δQ/T and the mechanical work done on a system when it expands is –PdV. • Substituting into • dG = dE + PdV +VdP – TdS – SdT • Leaves: dG = -SdT + VdP Apply equation to a closed system • Closed system contains pure substance – vapor – condensed phase • Phases co-exist in equilibrium. • Objective will be to see how the pressure of the vapor depends on the temperature of the system. Write the Free Energy Equation twice • Once for each phase • dGc = -ScdT + VcdP condensed phase • dGv = -SvdT + VvdP vapor phase c refers to the v refers to the Objective of the Exercise • Change system temperature • See how the pressure of the vapor changes • Note: – Both the vapor and condensed phase are in thermal equilibrium – Both are at the same temperature – System remains in thermal equilibrium if the molar free energy of the system, also known as the chemical potential, remains constant. Definition of chemical equilibrium between two phases • Free energy is the same in both phases • Gc = Gv • Changes in free energy when some independent variable is changed must be the same if they are to remain in equilibrium dGc = dGv • -ScdT + VcdP = -SvdT + VvdP • (Sv - Sc )dT = (Vv- Vc)dP • (Sv - Sc ) is the entropy change that takes place when material moves from the condensed phase to the vapor • ΔS = ΔQ/T where ΔQ is the amount of heat required per mole of material moved between the phases • ΔQ is just the heat of vaporization! • dP/dT = (Sv – Sc)/(Vv – Vc) = ΔHv/(TΔV) • This is the Clapeyron equation (E. Clapeyron, J. ẻcole polytech. (Paris) 14(23), 153 (1834). It relates the change in pressure of a vapor to the temperature in a closed, mono-component system to the heat of vaporization, system temperature and molar volume change of the material on vaporization. Creating of an Ideal Gas • For lack of a better model, we treat most vapors as ideal gases, whose molar volume is given by: • V/n = RT/P • Alternatively, equation of state is needed • Molar volume of gas is typically factor of 500 larger than condensed phase • Hence Vc is negligible in comparison Substituting and Integrating • dP = (ΔHv/Vv)dT/T = (PΔHv/RT)dT/T • dP/P = ΔHv/R)dT/T2 • ln(P(T)/ P0) = -(ΔHv/R)(1/T – 1/T0) • P(T) = P0 exp(-ΔHv/R(1/T – 1/T0)) • The vapor pressure in equilibrium with a condensed phase increases exponentially (sort of: exp(-1/T) isn’t exactly an exponential!) with temperature from zero up to the critical temperature. • Borne out in the vapor pressure charts and generating functions for them. • Deviations from linearity on the log-log plot – Temperature dependence of the heat of vaporization – exp (-1/T) isn’t really linear in the exponent. Heat of Vaporization from CRC Data Log10p(Torr) = -0.2185*A/T + B Vapor Pressure of Water Vapor Pressure (Torr) 10000 "Normal boiling point" 1000 100 10 1 0.1 -20 0 20 40 60 Temperature (C) 80 100 120 Generating Function s Imperfect • Normal boiling point of water on the plot is about 108.2 C. • I cheated; I looked at the data! • What does “Normal boiling point” mean?? • What does “boiling” mean??? Kubaschewski, Evans and Alcock, Metallurgical Thermochemistry, Pergammon, Oxford (1967) • Log10p(Torr) = A/T + BlogT + CT + D Vapor Pressure of Water Vapor Pressure (Torr) 10000 "Normal boiling point" 1000 100 10 Kubaschewski et al. 1 0.1 -20 0 20 40 60 Temperature (C) 80 100 120 From Kubaschewski’s Data • • • • Vapor pressure at 100 C is 758 Torr Much better approximation! Caveat emptor! “Life is like a sewer: the quality of the fit depends on the number of terms in the generating function!” RCA Charts • Great resource tool: the “RCA Charts”, which live in 327 JFB. Treat them with great respect: they are much older than you are and irreplaceable. • “Often imitated • Never duplicated” Other Sources of Data • Vapor Pressures of Pure Substances, Daniel R. Stull, Industrial and Engineering Chemistry, April 1947, pp. 517 – 550. (Tables give data on temperature required to generate a specific vapor pressure.) • Vapour Pressure of the Elements, An. N. Nesmeyanov, Academic Press, NY, NY (1963). Gibbs Phase Rule • • • • • • • F=C–P+2 F = number of degrees of freedom C = number of components P = number of phases What is a phase? What is a component? What is a degree of freedom? Phase Diagram of Water Melting curve Boiling curve “Normal boiling point” “Melting point” Sublimation curve Homework 1. Determine the vapor pressure at 77 K – Water – Carbon monoxide 2. What is the boiling point of water in a vacuum system at 10-6 Torr? 3. What is the vapor pressure of carbon dioxide at room temperature? 4. What is the condensed phase of carbon dioxide in equilibrium with the vapor at room temperature? How do you know?