L7b-1
Review: Fixed-Volume CSTR Start-Up
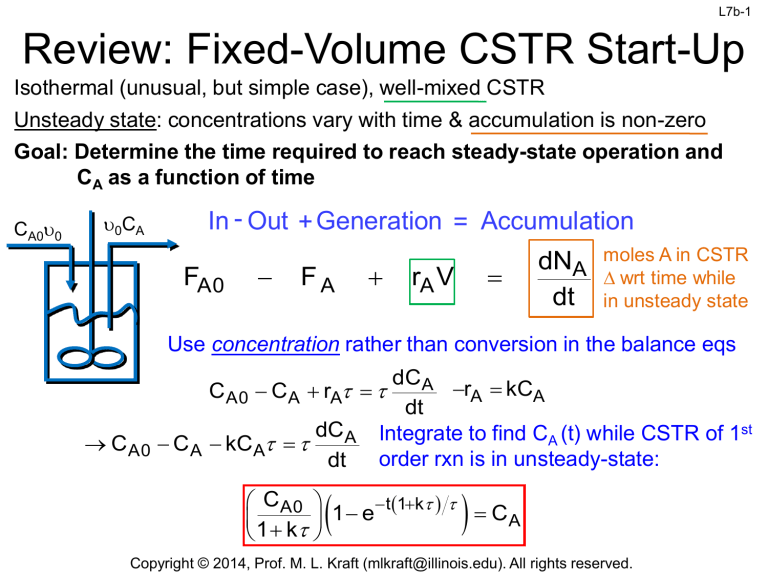
Isothermal (unusual, but simple case), well-mixed CSTR
Unsteady state: concentrations vary with time & accumulation is non-zero
Goal: Determine the time required to reach steady-state operation and
CA as a function of time
CA0u0
u0CA
In - Out + Generation = Accumulation
FA0
FA
rA V
dNA
dt
moles A in CSTR
D wrt time while
in unsteady state
Use concentration rather than conversion in the balance eqs
dC A
rA kCA
C A0 CA rA
dt
dCA Integrate to find CA (t) while CSTR of 1st
CA0 CA kCA
order rxn is in unsteady-state:
dt
CA0
t 1k
1
e
CA
1 k
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-2
Review: Time to Reach Steady-State
C A0
At steady state, CA0
t 1k
CAS
1 e
CA
1 k
t is large and: 1 k
0
In the unsteady state, CA0
CA0
t s 1k
0.99
1 e
when CA = 0.99CAS:
1
k
1 k
4.6
t time to reach 99% (CA = 0.99CAS) of
steady-state concentration in terms of k
1 k
When k is very small
t 4.6
(slow rxn), 1>>k: s
When k is very big
(fast rxn), 1<<k
63% of the steady-state
concentration is achieved at: 1 k
ts
4.6
k
CA = 0.63CAS
99% of the steady-state
4.6
concentration is achieved at:
1 k
CA 0.99CAS
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Enhanced Yield in SemiBatch Reactor
F
Semi-batch
L7b-3
D
FB
A
V0 + u0t
A+B
A+B
→P
V0
V0 + u0t
A+B⇌
C+D
V0 - u0t
Vf
Scenario 1 shown in blue. Scenario 2 shown in red.
Scenario 1: Enhance selectivity of desired product over undesired side product
Higher concentrations of A favor formation of the desired product
Higher concentrations of B favor formation of the undesired side product
Scenario 2: Improve the product yield obtained from a reversible reaction
A l B l
C l D g
Allowing D(g) to bubble out of solution pushes equilibrium towards completion
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
CBu0
Review: Mole Balance on A for
Semi-Batch Reactor
L7b-4
Goal: Find how CA D with time (assume reactor is well-mixed)
In - Out + Generation =
Accumulation
dNA
0 0 rA V
dt
Use whatever units are most convenient (NA, CA, XA, etc)
V0 + u0t
Convert NA to CA using:
NA
dCA V r V V dCA C dV
CA NA CA V
A
A
rA V
dt
dt
V
dt
Reactor volume balance:
In - Out + Generation = Accumulation
d V u = u0
dV
u0
0u0 0 0
V0 u0t V
0
dt
dt
rA V V
dCA
C Au0
dt
Rearrange to get in
terms of dCA/dt
rA
CAu0 dCA
V
dt
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Mole Balance on B in
Semi-Batch Reactor
C u
L7b-5
Goal: Find how CB D with time (assume reactor is well-mixed)
B 0
Mole balance on B:
In - Out + Generation =
FB0
V0 + u0t
0 + rB V
dNB
rB V FB0
dt
Accumulation
dNB
dt
NB CB V
dC
d
dV
CB V rB V CB0u0 CB V B rB V CB0u0
dt
dt
dt
dV
u0
Substitute
dt
dC
CBu0 V B rB V CB0u0 Rearrange to get in terms of dCB/dt
dt
u0 CB0 CB
dCB
rB
dt
V
Balance on B
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-6
CBu0
Review: Semi-Batch Mole
Balances in Terms of NA
Goal: Find how NA & NB D with time (reactor is well-mixed)
In - Out + Generation = Accumulation
0
0
rA V
dNA
dt
dNA
rA V
dt
V0 + u0t
N
C
Substitute: -rA = kACACB and V0 u0 t V and
V
NANB
dNA
dNA
NANB
then rA k
r
V
k
A
2
dt
dt
V0 u0 t
V u t
0
0
NB comes from basic mole balance:
dNB
NANB
dNB
k
FB0
rA V FB0
dt
V0 u0 t
dt
The design eq in terms of XA can be messy. Sometimes it gives a single
equation when using Nj or Cj gives multiple reactor designs
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
2A→B -rA = kCA2
α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
1.
Mole balance
2.
Rate law
dX A
r 'A
dW
rA kC A 2
FA0
3. Stoichiometry (put CA C A
in terms of X)
4. Combine
C A0 1 X A P
1 X A P0
dX A
dW
5. Relate P/P0 to W
L7b-7
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
dX A
dW
k C A02
1 XA 2 P 2
P0
1 X A
2
2
kCA0 1 X A P
u0 1 X A 2 P0
FA 0
2
dP
T P0
1 XA
dW
2 T0 P P0
1
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
2A→B -rA = kCA2
α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
4. Combine
5. Relate P/P0 to W
L7b-8
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
dX A kCA0 1 X A P
dW
u0 1 X A 2 P0
dP
P0
1 X A
dW
2 P P0
2
2
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
First, need to determine , CA0, & u0.
NTf NT0
1 2
0.5
NT0
2
What is CA0?
P0
20atm
mol
C A0 0.813
CA0
RT0
dm3
dm3 atm
0.082
300K
mol K
FA0
10mol min
dm3
u0
u0 12.3
3
C A0
min
0.813mol dm
P0 V0 NT0RT0
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
2A→B -rA = kCA2
α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
L7b-10
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2
dP
P0
dX A kCA0 1 X A P
1 X A
2
dW
2 P P0
dW
u0 1 X A P0
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
3
mol
dm
0.5
C A0 0.813
u0 12.3
dm3
min
2
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
2A→B -rA = kCA2
α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
L7b-11
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2
dP
P0
dX A kCA0 1 X A P
1 X A
2
dW
2 P P0
dW
u0 1 X A P0
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
3
mol
dm
0.5
C A0 0.813
u0 12.3
dm3
min
2
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion and P is L7b-12
measured at the outlet of the
PBR? The rxn is isothermal at
300 K, assume ideal gas
behavior, and the feed contains
pure A (g).
P = 14.28 atm
X = 0.93
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a L7b-13
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
F X
CSTR design eq: W A0 A
r 'A
FA0 X A
W
kC A0 1 X A
Use info from PBR to determine FA0, CA0 & k
dX A r ' A
dX A kC A
C 1 XA P T0
NT NT0 1 1
0
CA A0
dW
FA0
dW
FA0
NT0
1
1 XA P0 T
Do not plug in P and P0 that occurred in PBR
0
1
yet! Use Ergun eq to get P/P0 as a function of
Use PBR expt
W, plug into design eq & integrate over W!
P
1 W parameters to
Isothermal and =0. Ergun eq for P/P0 becomes:
solve for α
P0
9atm
1 1000kg
20atm
0.2025 1 1000kg
0.0008 kg1
P
0.0008
C
C
1
X
C
C
1
X
1
W
Plug into CA: A
A0
A
A
A0
A
kg
P0
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-14
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
F X
CSTR design eq: W A0 A
r 'A
FA0 X A
W
kC A0 1 X A
Use info from PBR to determine FA0, CA0 & k
dX A kC A
0.0008
CA CA0 1 XA 1
W
dW
FA0
kg
0.0008
k CA0 1 X A 1
W Rearrange
kg
dX A
dW
FA0
Plug CA into PBR
design eq:
dX A kC A0
0.0008 Integrate so that we can
W
1 X A 1
dW
FA0
kg get values of unknowns
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-15
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
F X
CSTR design eq: W A0 A
r 'A
FA0 X A
W
kC A0 1 X A
dX A kC A 0
0.0008
PBR design eq :
W
1 X A 1
dW
FA0
kg
X A dX
kC A0 W
0.0008
A
W dW
1
FA 0 0
kg
0 1 X A
1000kg
3
0.0008
1 kC A0
2
2
ln
1
1
W
F
3 0.0008 kg
1
X
kg
A
A0
0
1000kg
3
1
1
0.0008
2
kC A 0 2
ln
1
1
W
1
0.141
F
3
0.000
8
kg
kg
A0
0
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-16
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
F X
CSTR design eq: W A0 A
r 'A
FA0 X A
W
kC A0 1 X A
dX A kC A 0
0.0008
PBR design eq :
W
1 X A 1
dW
FA0
kg
1000kg
3
1
0.0008
2
1 kC A0 2
ln
1
1
W
kg
0.859 FA0 3 0.0008 kg
0
3
3
kC A 0
2
0.0008
2
0.0008
0.152
833.3 kg 1 1
1000kg 833.3 kg 1 1
0k g
FA0
kg
kg
3
kC A0
kC A0
833.3 kg 1 0.0894
0.152
833.3 kg 1 1 0.8 2 833.3 kg 1 1 0.152 F
FA 0
A0
kC A0
0.152
758.8kg
FA0
2.0 10 4 kg1
kC A0
FA0
Plug this value into
the CSTR eq
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-17
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
F X
CSTR design eq: W A0 A
r 'A
FA0 X A
W
kC A 0 1 X A
2.0 10 4 kg1
kC A0
FA0
XA
1000kg
2.0 10 4 kg1 1 X A
1
0.2
XA
0.2 0.2X A X A 0.2 1.2XA 0.17 XA
1 XA
Conversion in fluidized CSTR, no pressure drop
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
kg cat min atm
L7b-18
at 300K with E=80 kJ/mol, elementary rxn
How many kg of catalyst is required to achieve XA = 0.8?
Fluidized
CSTR
design eq:
F X
C u X
W A0 A W A0 0 A
r 'A
r 'A
1. What is CA0?
CA0 y A0CT0
y A0 =
Known: u0 and XA
Unknown: CA0 & -r’A
NA0
NT0
NT0
P
CT0
V
RT
Feed is a stoichiometric mixture
1
1
y A0 =
→ 1 part A, 2 parts B
1 2 3
6atm
P C 1
mol
C A0 y A0
A0
C
0.055
A0
3
3
RT
atm dm3
dm
0.082
443K
mol K
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-19
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
kg cat min atm
at 300K with E=80 kJ/mol, elementary rxn
How many kg of catalyst is required to achieve XA = 0.8?
F X
C u X
W A0 A W A0 0 A
r 'A
r 'A
2. What is –r’A? Units on k are:
r 'A kPA PB2
Ci
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
mol
kg cat min atm3
2a. What is PA?
Ni
P
i
V RT
Substitute for Cj & Cj0
Express rate law in terms
of partial pressure, not Cj
C j0 j j X A
For ideal, isobaric,
Cj
isothermal rxn:
1 XA
Pj0
j j XA
Pj
RT
RT
1 XA
Pj0 j j X A
Pj
1 XA
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-20
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
kg cat min atm
at 300K with E=80 kJ/mol, elementary rxn
How many kg of catalyst is required to achieve XA = 0.8?
F X
C u X
W A0 A W A0 0 A
r 'A
r 'A
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
2 Units on k necessitate expressing rate law in
2. What is –r’A? r 'A kPAPB
terms of partial pressure, not Cj
2a. What is PA?
A=-1
A=1
PA0 y A0PT0
Pj
Pj0 j j X A
1 XA
NT NT0
y A0
NT0
J
Fj0
FA0
C j0u0
CA0u0
C j0
CA0
1
3
y j0
y A0
y A0 (1 2 1)
1
PA0 6atm PA0 2atm
3
2
3
2atm 1 X A
PA
1 2 3 XA
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
kg cat min atm
L7b-21
at 300K with E=80 kJ/mol, elementary rxn
How many kg of catalyst is required to achieve XA = 0.8?
F X
C u X
W A0 A W A0 0 A
r 'A
r 'A
2. What is –r’A? r 'A kPAPB
2b. What is PB?
PA0 j j X A
PB
1 XA
2
3
2
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
PA
B=-2
PB
2atm 1 X A
1 2 3 XA
B
J
Fj0
FA0
C j0
CA0
FB0 2
2
FA0 1
2atm 2 2X A
4atm 1 X A
PB
1 2 3 XA
1 2 3 XA
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
y j0
y A0
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
L7b-22
at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
How many kg of catalyst is required to achieve XA = 0.8?
FA0 XA
CA0u0 XA
W
W
r 'A
r 'A
2
2. What is –r’A? r 'A kPAPB
2c. What is k at 443K?
k 443K
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
PB
4atm 1 X A
1 2 3 XA
k 443K k 300K e
PA
2atm 1 X A
1 2 3 XA
E 1 1
R T1 T2
1
80000J mol 1
8.314J molK 300K 443K
e
mol
53
kgcat min atm3
k 443K 1.663 106
mol
kgcat min atm3
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
L7b-23
at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
How many kg of catalyst is required to achieve XA = 0.8?
FA0 XA
CA0u0 XA
W
W
r 'A
r 'A
2
2. What is –r’A? r 'A kPA PB
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
4atm 1 X A
1 2 3 XA
mol
6
PB
k 443K 1.663 10
PA
2atm 1 X A
1 2 3 XA
kgcat min atm3
2atm 1 X A 4atm 1 X A
mol
6
r 'A 1.663 10
3 1 2 3 X
1
2
3
X
kgcat
min
at
m
A
A
1 X 3
mol
A
r ' A 1.663 106
32atm3
3
3
1 2 3 X
kgcat min atm
A
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
2
A + 2B → C
Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
k 53
mol
3
kg cat min atm
at 300K with E=80 kJ/mol, elementary rxn
How many kg of catalyst is required to achieve XA = 0.8?
C A0u0 X A
CA0 =0.055 mol/dm3
W
r 'A
1 X 3
mol
A
r ' A 1.663 106
32atm3
3
3
1 2 3 X
kgcat min atm
A
mol dm3
50
0.8
0.055
3
mi
n
dm
W
1 0.8 3
mol
6
32atm3
1.663 10
3
3
1 2 3 0.8
kgcat min atm
W 5.24 10 7 kg cat
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
L7b-24









