The Mole Concept and Avogadro's constant
advertisement

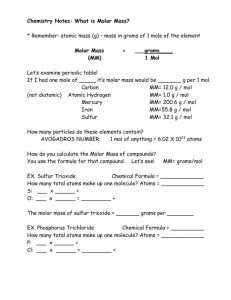
The Mole Concept and Avogadro's constant The Mole-at first glace What is “the mole”? Dictionary definition: “a small burrowing mammal with dark velvety fur, a long muzzle, and very small eyes, feeding mainly on worms, grubs, and other invertebrates… Animal?- must be biology-not chemistry! Are we in the right class? The Mole-demystified Another definition-”Amount of substance…”- more like chemistry! Pair of socks (2) Dozen of eggs (12) Ream of paper (500) The mole & coins! Suppose you had to count a vast number of coins What is the quickest method? If the mass of a certain number of coins is known, the total number can be weighed and then the amount can be calculated. The Mole-demystified 2 Quantity of 6.02x1023 Why this magic number? It is the number of particles present in exactly 12 grams of the isotope carbon-12 Called the Avogadro Constant The mole If you weigh out 12g of carbon-12, you will get 6.02x1023 of carbon atoms The mole All elements measured relative to carbon-12 Amount of substance (mol)= number of particles/6.02 x 1023mol-1 Number of particles= amount of substance (mol) x 6.02 x 1023mol-1 Mole is the same number regardless of the representative particle: atom, molecule, ion, electron The mole Example 1 Calculate the number of molecules of water in 0.01mol of water Number of water molecules=0.01mol x 6 x 1023 mol-1 = 6 x 1021 How many carbon atoms are there in 0.02 mol of carbon dioxide? How many oxygen atoms are there in 0.02 mol of carbon dioxide? Molar Mass Molar mass is the mass of 1 mole of the substance Number of moles=mass in g/mass of 1 mole in g A mole of carbon atoms, C, has a mass of 12g A mole of hydrogen atoms, H, has a mass of 1g BUT a mole of hydrogen molecules has a mass of 2g Molar mass- example Calculate the molar mass of aluminium sulfate, Al2(SO4)3 2 x Al= 2 x 26.98g/mol= 53.96g/mol +3 x S= 3 x 32.06g/mol= 96.18g/mol +12 x O= 12 x 16.00g/mol=192.00g/mol Molar mass= 342.14g/mol Relative atomic mass, relative formula mass Relative atomic mass- the weighted average mass (according to relative abundances) of all the naturally occurring isotopes of an element compare with an atom of the carbon-12 isotope, which has a mass of exactly 12 Formula= 12 x average mass of one atom of the element/mass of one atom of carbon-12 Relative molecular mass is the sum of the relative atomic masses of all the atoms in 1 molecule. Relative molecular mass=12 x average mass of one molecule of the element/ mass of one atom of carbon-12 Molar mass vs relative atomic/molecular masses Same except molar mass has units of g/mol Relative atomic mass & relative molecular mass are dimensionless Find the molar mass of magnesium carbonate, MgCO3 MgCO3= 24+12+3x16=84g/mol Find the relative molecular mass of carbon dioxide, CO2. CO2= 12+2x16=44 (NO UNITS!) Amount of substance vs mass More problems Find the moles of water molecules present in 54g of water Moles of water molecules= 54g/18g mol-1=3.0mol Everything combined! Calculate the number of calcium atoms present in 0.5kg 0f calcium Amount of calcium=500g/40g mol-1=12.5mol Atoms of calcium=12.5 mol x Avogadro's constant= 12.5 x 6.02 x 1023= 7.53 × 1024 Everything combined more! Calculate the number of carbon and hydrogen atoms and the total number of atoms in 22g of propane, C3H8. Molar mass of propane, C3H8=(3x12)+(8x1)=44g mol-1 1 mole of propane therefore has a mass of 44g and contains 6.02x1023 molecules of propane. Amount of propane=22g/44g mol-1= 0.50mol Molecules of propane=0.5 x 6.02x1023=3x1023 Total number of atoms=11x3x1023=3.3x1024 Total number of carbon atoms=3x3x1023=9x1023 Total number of hydrogen atoms=8x3x1023=2.4x1024