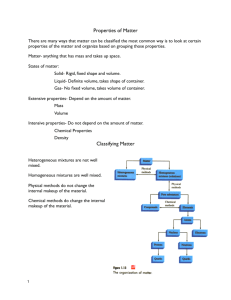
Ch. 1 Outline
advertisement

Honors Chemistry Mrs. Klingaman Chapter 1: Matter and Measurement Objectives: The students will be able to… Define chemistry and understand why it is useful to study chemistry Define matter, including states of matter Distinguish between pure substances and mixtures Characterize matter and identify methods of separation of matter Understand and apply the process of the scientific method Understand the units of measurement used in science, including metric units and SI units of measure Distinguish between qualitative and quantitative data apply significant figures to account for inherent uncertainty in measured data Apply dimensional analysis (unit conversions) and conversion factors to scientific analysis Name: _____________________________________ Mods: ______________ Honors Chemistry Reading Guide Chapter One: Matter and Measurement 1.1 – The Study of Chemistry 1. Chemistry- 2. Matter- 3. Element- 4. Atoms- 5. Molecules- 6. Why study Chemistry (list some examples/applications)? . . . 1.2 – Classifications of Matter 7. States of Matter- Gas- Liquid- Solid8. Pure Substance Element- Compound- 9. Law of Constant Composition: 10. Mixture Heterogeneous- Homogeneous- (aka: ______________________) 1.3 – Properties of Matter 11. Physical properties- 12. Chemical properties- 13. Intensive properties- 14. Extensive properties- 15. Physical changes- 16. Chemical changes17. Separation of Mixtures Filtration- Distillation- Chromatography- 18. Scientific Method: identify the key features to this process 1.4 – Units of Measurement 19. SI UnitsPhysical Quality Mass Length Time Temperature Name of Unit Abbreviation Amt. of a substance Electric Current Luminous intensity Temperature conversions: Kelvin/Celsius- Celsius/Fahrenheit- 20. Derived SI Units Density- Volume1.5 – Uncertainty in Measurement 21. Uncertainty in Measurement Exact numbers- Inexact numbers- 22. Precision- 23. Accuracy- 24. Significant figures Rule #1- Rule #2- Rule #3- 25. Significant figures in calculations Multiplication & division- Addition & subtraction- 1.6 – Dimensional Analysis 26. Dimensional analysis and Conversion factors given unit x desired unit = desired unit given unit Give one full example of dimensional analysis with real numbers and units!