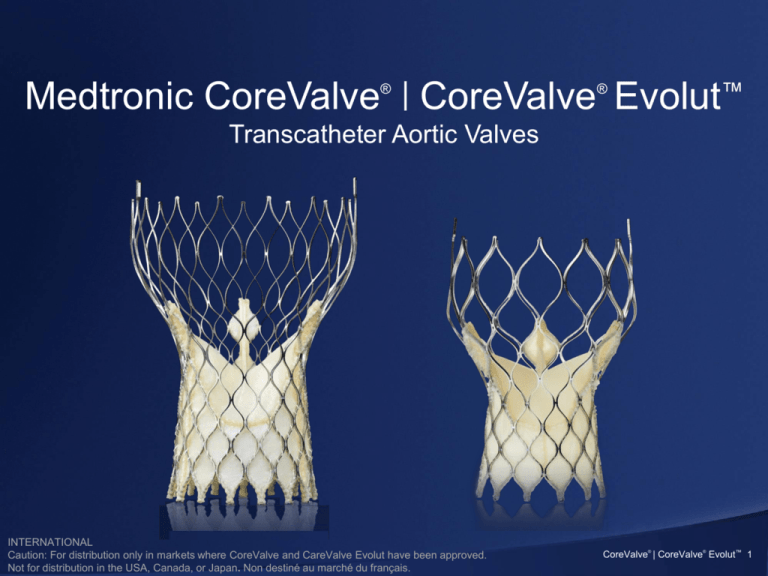
Medtronic CoreValve | CoreValve Evolut™
®
®
Transcatheter Aortic Valves
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 1
Clinical Challenges in TAVI
Clinical Need
1
Access, even in patients with
• Low delivery profile
small or challenging vasculature • Alternative—non-femoral—access
Positioning, even in angulated
2 or otherwise challenging
anatomies
3
Design Challenge
Conformability to a wide range
of patient anatomies—annulus
size, shape, calcification
Durability long-term with
4 hemodynamic and structural
integrity
• Step-wise deployment with ability adjust
and refine valve position
• Full range of valve sizes
• Conformability at the annulus with
circularity at level of valve function
• Optimal tissue selection for strength,
pliability and thinness
• Frame designed to reduce leaflet stress
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 2
System Components
Valve
Loading System
Delivery System
Self-expanding
Nitinol frame with
porcine pericardial
valve
Disposable cones
and tubes used to
compress the valve
True 18Fr catheter
delivery system with
AccuTrak® stability
layer
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 3
CoreValve and CoreValve Evolut Valves
31mm
29mm
26mm
CoreValve
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
23mm
CoreValve Evolut
CoreValve® | CoreValve® Evolut™ 4
• Anatomical Fit
• Deliverability
• Performance & Durability
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 5
CoreValve Design Legacy
Outflow
Supports valve commissures and
enables controlled deployment
Low Radial Force
Waist
Promotes circularity, supports
supra-annular valve
High Hoop Strength
Inflow
Conforms and seals to the
annulus
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
High Radial Force
CoreValve® | CoreValve® Evolut™ 6
Supra-Annular Valve Design
Flexible frame conforms to native annulus shape while maintaining the
bioprosthesis in a higher position
– This decoupling of the valve from native annulus shape minimizes the
impact of ellipticity at the valve level post deployment1
Patient 1
Patient 2
Patient 3
Images courtesy of Drs. de Jaegere and Schultz, Erasmus MC,
1. Data on file at Medtronic
Rotterdam, The Netherlands
INTERNATIONAL
CoreValve | CoreValve Evolut™ 7
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
®
®
CoreValve Evolut
Adds TruFit Technology
Optimizes coaptation
in non-circular anatomy with
supra-annular valve position
Customizes anatomical fit
via a tailored height and
shape
Conforms to the anatomy
and promotes sealing
with optimized interference
and radial force
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 8
CoreValve Evolut
Tailored Height and Shape
Reduced height
Reduce height of outflow for
better fit, especially in
angulated anatomies
45 mm
12 mm
Preserved skirt length
Preserved coronary access
Maintains CoreValve cell
geometry for coronary access
Provides seal against
paravalvular leak
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 9
CoreValve Evolut
Optimized Interference and Radial Force
Optimized
Performance
More consistent
interference and
radial force across
annular size range
while maintaining
conformability
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 10
Full Range of Valve Sizes
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 11
Use in failed surgical bioprostheses
Now CE Approved
Low post-procedural gradients
1
Valve Inflow
Leaflets sit above the annulus
where the frame is least
constrained, opening up the
valve for greater flow
Leaflets
CoreValve
1. Dvir et al., TCT. Miami, Fl. Oct 2012
Large potential orifice area
Surgical Valve
Aortic Annulus
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 12
• Anatomical Fit
• Deliverability
• Performance & Durability
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 13
AccuTrak® Delivery System
AccuTrak® Stability Layer
15Fr
12Fr
7cm
18Fr
Over-the-wire 0.035 compatible
6 mm
Low-Profile Access
Stable Deployment
Slow, Controlled Release
with true 18Fr profile
across all valve sizes
with AccuTrak Stability
Layer
with partial repositionability
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 14
Clinical Experience with AccuTrak
• Implants at a depth of 4-6
mm minimize paravalvular
leak
N=134
• Example: 134 CoreValve
patients treated at 2
experienced centers1
• All implants performed
using the AccuTrak delivery
system
1.Tchetche, et al., EuroIntervention 2012; e-publication
Patients (%)
Procedural Success
133 (99.2)
BAV
129 (98.5)
Balloon-Annulus Ratio
0.95 0.09
Depth of Implant
4.9 2
PVL > 2/4
0 (0)
Central leak
0 (0)
New LBBB
18 (13.4)
New Transient or
Sustained AVB
17 (12.7)
New Pacemaker
Implantation
12* (10.6)
*12 of 113 patients without baseline pacemaker.
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 15
Approved Access Routes
Direct Aortic
Subclavian
Transfemoral
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 16
• Anatomical Fit
• Deliverability
• Performance & Durability
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 17
Characteristics of Performance
Valve Design
Leaflet Geometry
Supra-annular
Tissue Selection
Thickness
Tensile Strength
Pliability
Tissue
Treatment
Anti-calcification
Performance
Clinical Outcomes
Bench Testing
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 18
Frame Material Selection—Nitinol
Superelasticity
•Compact designs
and small delivery
systems
Shape Retention
• Self-anchoring
• Controlled
retraction for
precise delivery
and placement
• Maintain valve
shape
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
Proven
Performance
•Resistant to corrosion
•Low thrombogenicity
•Conformable to
patient anatomy
•Fatigue performance
CoreValve® | CoreValve® Evolut™ 19
Porcine pericardium is the optimal tissue for
valve performance and low-profile delivery
Thin
Strong
1.
2.
3.
4.
Porcine pericardium thickness is
about half that of bovine. Thinner
tissue prevents tissue damage during
crimping, tracking, and deployment,
allowing for low-profile delivery
across all valve sizes.1,2
Porcine Pericardium
Bovine Pericardium
The ultimate tensile strength (UTS)
and suture pull out stresses for
porcine and bovine pericardium are
not statistically different1,3 and peak
physiologic stresses are significantly
less than both UTS values4
Sacks MS. Uniaxial mechanical and structural properties of bovine versus porcine pericardial tissue. Medtronic
Engineered Tissue Mechanics Laboratory. University of Pittsburgh, Pittsburgh, PA. January 17, 2008. Data on File.
Braga-Vilela AS, Pimentel ER, Marangoni S, Toyama MH, de Campos Vidal B. Extracellular matrix of porcine
pericardium: Biochemistry and collagen architecture. J Membr Biol. 2008 Jan;221(1):15-25.
Garcia Paez JM, Carrera A, Herrero EJ, et al. Influence of the selection of the suture material on the mechanical
behavior of a biomaterial to be employed in the construction of implants.
Part 2: porcine pericardium. J Biomater Appl. 2001;16:68-90.
Li, K and Sun, W. “Simulated thin pericardial bioprosthetic valve leaflet deformation under static pressure-only loading
conditions: Implications for percutaneous valves” Ann Biomed Eng. 2010 Aug;38(8):2690-701.
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 20
Commissure height and deep leaflet cuts
minimize leaflet stress
• Finite element analysis of the CoreValve® leaflets demonstrate a 12%
reduction in stress when compared to traditional valve designs
• Areas of high stress can induce collagen degeneration that over time could
lead to tearing and valve failure1
• Valve designs that reduce leaflet stresses “are likely to have improved
performance in long-term applications”2
1.
2.
Schoen Frederick J. Cardiac Valve Prostheses: Pathological and Bioengineering Considerations. J Cardiac Surg. 1987;2:65-108.
Sun W., Li K., Sirois E. Simulated elliptical bioprosthetic valve deformation: Implications for asymmetric transcatheter valve deployment. J Biomech. 2010;43:3085-3090.
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 21
AOA® anti-mineralization treatment reduces both
early and late valvular calcification
•
•
•
Alpha-amino oleic acid (AOA®) treatment inhibits calcium formation on
prosthetic valve leaflets.
Unlike surfactants, AOA bonds with the tissue to block calcium binding.
AOA has 20 years of proven clinical success on Medtronic’s surgical valves.1
1. Medtronic Freestyle Aortic Root Bioporsthesis was first implanted clinically in August 1992.
INTERNATIONAL. CAUTION: For distribution only in markets where CoreValve® is approved. Not for distribution in U.S., Canada or Japan. © Medtronic, Inc. (2012), All Rights Reserved.
Potential Complications
Implantation of the CoreValve Transcatheter Valve may
include the following risks:
– Death including all cause and cardiovascular mortality
– Myocardial infarction including coronary occlusion
– Stroke including permanent stroke and TIA
– Re-intervention including sAVR and repeat valve placement
– Aortic regurgitation
– Permanent pacemaker placement
– Pericardial tamponade (wire perforations)
– Vascular and bleeding complications
– Valve migration or fracture
For complete list of adverse events, warnings and contraindications
reference CoreValve IFU
CoreValve® is a registered trademark of Medtronic CV Luxembourg S.a.r.l.
Evolut™, TruFit™, AccuTrak®, and AOA® are registered trademarks of Medtronic, Inc.
INTERNATIONAL
Caution: For distribution only in markets where CoreValve and CareValve Evolut have been approved.
Not for distribution in the USA, Canada, or Japan. Non destiné au marché du français.
CoreValve® | CoreValve® Evolut™ 23