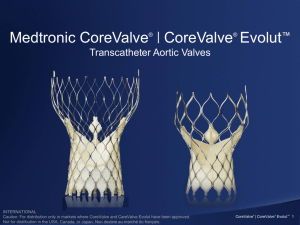
Supra-Annular Valve
advertisement

CoreValve® Durability and Valve Performance INTERNATIONAL INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Not approved in the USA, Canada or Japan. UC201202505c EE Medtronic Bioprosthesis Evolution Third Generation Product Features Hancock I 1970’s Second Generation First Generation • High Pressure Fixation • Large native muscle bar • Flexible Stent Posts Rigid Inflow • Low/ Zero pressure • Toluidine Blue, T6 • Flexible stent *CoreValve • Supra-Annular • Porcine Pericardium Freestyle • Physiologic Fixation • AOA • Flexible Stent Melody Hancock II 1990’s Mosaic • Pulmonic Transcatheter • Bovine Jugular Vein 1980’s Hancock MO Intact INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 12 Hancock II David and colleagues 94% INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 89.8% Mosaic ® INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Transcatheter Aortic Valves The Durability Challenge How do we go from here……….. to here INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. and never end up here Valve Durability: A Lesson from Surgical Valves Maintaining Proper Leaflet Motion is Critical to Long Term Valve Durability • Leaflet bending/folding during valve operation induces high stresses on leaflets. High bending stresses on leaflets can lead to bending fatigue and potentially delamination, calcification, and/or valve failure 1 • Misalignment, leaflet prolapse, asynchrony, poor coaptation, high commissure stress, pinwheeling/bending may lead to early failure. INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 1. Schoen 1987 Surgical Pericardial Valve Failures Current generation surgical pericardial bioprostheses reveal that these devices can exhibit SVD leading to explant as early as two to four years with continued risk of failure over time 1 Cosgrove D, Lytle B, Taylor P, et al. The Carpentier-Edwards Pericardial Aortic Valve Ten-Year Results. J Thorac Cardiovasc Surg. 1995;110:651-62. 2 Roselli E, Smedira N, Blackstone E. Failure Modes of the Carpentier-Edwards Pericardial Bioprosthesis in the Aortic Position J Heart Valve Dis 2006;15:421-8. INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. CoreValve Design + Tissue Selection = Durability and Performance Supra-annular valve design provides optimal leaflet coaptation Commissure height and deep leaflet cuts minimize leaflet stress Self-expanding nitinol frame enhances durability and deliverability Porcine pericardium is the optimal tissue for valve performance and low-profile delivery AOA® anti-mineralization treatment reduces both early and late valvular calcification* INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. *AOA anti-mineralization treatment will be available on CoreValve in Fall 2012. Frame Design Challenges Design Requirement Challenges to Durability Self-anchoring/Migration • Material capable of providing appropriate radial force over a range of sizes and shapes Delivery Profile • Frame capable of withstanding crimp profile Span a range of sizes with a single valve and accommodate anatomical variations • Frame capable of maintaining structural integrity spanning a range of sizes and shapes Withstand in-vivo loading • Frame capable of withstanding multiple modal cycle loading (radial, compression, torsion, etc.) INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Frame Fatigue Analysis & Testing • Rigorous analysis of CoreValve patient CT datasets provided definition of aggressive boundary condition input for FEA and fatigue tests • State of the art finite element (FEA) methods used to predict strain distribution within frame under combined loading modes • Frame fatigue testing successfully completed to 600M cycles (15yrs) under multiple test conditions to demonstrate frame durability Enhanced In Vivo Deformation Analysis from CT Data Combined Loading FEA INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 600M Cycle Frame Fatigue Testing Bench test results shown on this slide and throughout the presentation may not be indicative of clinical performance. Migration Conduit Development & Testing • Patient CT scans are studied to determine the most challenging anatomical conditions for the CoreValve frame • Test conduits are developed from materials designed to match the compliance of the human aorta • Valves are tested for migration resistance up to stage 3 hypertensive flow conditions INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Tissue Design Challenges Design Requirement Challenges to Durability Delivery Profile • Prevent tissue damage during crimping, tracking, and deployment Span a range of sizes with a single valve • Preserve long term leaflet function/motion Accommodate anatomical variations • Mitigate distortion at the valve level to maintain coaptation alignment and leaflet motion • Minimize redundancy that leads to extreme leaflet bending Withstand potential postimplant BAV • Post-implant BAV must not damage tissue INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Porcine pericardium is the optimal tissue for valve performance and low-profile delivery • The ultimate tensile strength (UTS) and suture pull out stresses for porcine and bovine pericardium are statistically equivalent1,2 9 8 7 • Peak physiologic stresses are significantly less than both UTS values3 6 MPa • Porcine pericardium thickness is about half that of bovine. Thinner tissue prevents tissue damage during crimping, tracking, and deployment, allowing for low-profile delivery across all valve sizes.1,4 5 4 3 2 Peak Physiologic Stress 1 1. 2. 3. 4. Sacks MS. Uniaxial mechanical and structural properties of bovine versus porcine pericardial tissue. Medtronic Engineered Tissue Mechanics Laboratory. University of Pittsburgh, Pittsburgh, PA. January 17, 2008. Data on File. Garcia Paez JM, Carrera A, Herrero EJ, et al. Influence of the selection of the suture material on the mechanical behavior of a biomaterial to be employed in the construction of implants. Part 2: porcine pericardium. J Biomater Appl. 2001;16:68-90. Li, K and Sun, W. “Simulated thin pericardial bioprosthetic valve leaflet deformation under static pressure-only loading conditions: Implications for percutaneous valves” Ann Biomed Eng. 2010 Aug;38(8):2690-701. Braga-Vilela AS, Pimentel ER, Marangoni S, Toyama MH, de Campos Vidal B. Extracellular matrix of porcine pericardium: Biochemistry and collagen architecture. J Membr Biol. 2008 Jan;221(1):15-25. INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 0 Porcine Bovine AOA® anti-mineralization treatment reduces both early and late valvular calcification • • • Alpha-amino oleic acid (AOA) treatment inhibits calcium formation on prosthetic valve leaflets. Unlike surfactants, AOA convalently bonds with the tissue to block calcium binding. AOA has 20 years of proven clinical success on Medtronic’s surgical valves.1 Non-treated Tissue Glutaraldehyde preserved tissue AOA® Treated Tissue The likelihood of calcification increases on nontreated tissues Tissue Glutaraldehyde Matrix AOA covalently bonds to the free aldehyde groups in the glutaraldehyde tissue AOA INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Blood Cell AOA reduces both early and late valvular calcification Calcium 1. Medtronic Freestyle Aortic Root Bioporsthesis was first implanted clinically in August 1992. *AOA anti-mineralization treatment will be available on CoreValve in Fall 2012. Valve Height Reduces Leaflet Stresses • Areas of high stress can induce collagen degeneration that over time could lead to tearing and valve failure1 • Valve designs that reduce leaflet stresses “are likely to have improved performance in long-term applications”2 17 mm tall 14 mm tall 12% Lower Stress in the Taller Valve 1. Schoen Frederick J. Cardiac Valve Prostheses: Pathological and Bioengineering Considerations. J Cardiac Surg. 1987;2:65-108. 2. Sun W., Li K., Sirois E. Simulated elliptical bioprosthetic valve deformation: Implications for asymmetric transcatheter valve deployment. J Biomech. 2010;43:3085-3090. INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Finite Element Analysis (FEA) Demonstrates Reduction in Leaflet Stresses Over Traditional Valve Designs1 120 mm Hg of Pressure is Applied to Both Valves Max Stress = 1.34 MPa 1. Data on file at Medtronic INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Max Stress = 1.50 MPa Supra-Annular Valve Design Can Mitigate Impact of Elliptical or Undersized Deployment • Flexible frame conforms to native annulus shape while maintaining bioprosthesis in a higher position • Decoupling of valve from native annulus minimizes ellipticity at the valve level 1 INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. 1. Data on file at Medtronic Crimping For Up To 60 Minutes Does Not Damage the CoreValve Tissue • CoreValve bioprostheses (n=8) were crimped and loaded according to the IFU for 15, 30, 45 and 60 minutes • TCV leaflets were inspected under microscope, then removed from the frame and sectioned for histologic analysis • Microscopic inspection showed faint imprints that rehydrated quickly during the course of inspection. No evidence of damage was seen on the sutures, leaflets, or skirt tissues • Histologic inspection showed mild indents, but these were not correlated to impression sites and were also found on control leaflets These indents do not correspond to a site of observed imprints cut MOA H1 FM H2 section sectionF1 F1 These indents are in the area of the observed imprints cut MOA FM INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. section sectionF2 F2 Medtronic has Performed Rigorous Testing to Confirm the Safety of Post-Implant BAV • In two separate studies, CoreValve bioprostheses were deployed in synthetic and tissue conduits that were modeled after calcified human aortic anatomy • Balloon dilatation was performed using 25mm and 28mm NuMed, Z-Med II balloons at maximum-rated burst pressures for 5 seconds • Valves were inspected immediately after removal of the balloon, then assessed for hydrodynamic performance on a pulse duplicator INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Post-Implant Balloon Dilatation Does Not Damage CoreValve’s Tissue, Frame, or Sutures Conclusions • Inspections showed no evidence of damage to either the valve tissues or assembly sutures in either study • The hydrodynamic performance data showed that there was no significant difference in EOA between pre and post balloon dilatation Pre Balloon Dilatation INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Post Balloon Dilatation Performance • Accelerated wear testing (AWT) was performed on 24 valves in both circular and elliptical configurations – Valves were cycled for 200 million cycles (5 years) – Result: Minimal valve wear and low stable gradient and regurgitation levels Circular Deployment INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Elliptical Deployment 1.35 aspect ratio Tissue Integrity • Histology performed on leaflet samples after 200 million cycles in an elliptical configuration – All test samples were crimped and loaded into the delivery system prior to wear testing – Leaflets showed no evidence of internal leaflet delamination or collagen loss – Laser-cut free margins were fully intact1 outflow inflow Figure 1. Leaflet as received 1. Data on file at Medtronic Figure 2. Slices submitted for histopathology INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. Figure 3. The pericardial leaflet section is intact Conclusions • There have been over 27,000 CoreValve device implants in over 50 countries since the first implantation in 2004 • No frame fractures have been reported in an implanted CoreValve device to date • In AWT, CoreValve device demonstrated a consistently low regurgitant fraction and stable EOA from base line to post-200 million cycles • Long-term (3 and 4-year) durability is indicated by the absence of structural valve deteriorations and sustained hemodynamic performance INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan. CoreValve is a registered trademark of Medtronic CV Luxembourg S.a.r.l. AOA is a registered trademark of Medtronic, Inc. INTERNATIONAL Caution: For distribution only in markets where CoreValve has been approved. Not approved in the USA, Canada or Japan.