Medtronic CoreValve® System Summary of
Clinical Experience
UC201402534 EE
Agenda
• Background on aortic stenosis (AS) and introduction to
transcatheter aortic valve implantation (TAVI)
• Overview of the CoreValve clinical portfolio
• CoreValve clinical performance
– All-cause Survival
– Efficacy
– Other Outcomes
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Background
• Aortic Stenosis (AS) is the most prevalent native valve disease1
• More than 300,000 patients have severe AS worldwide
• The mortality rate for untreated severe AS is up to 50%-60% within 2
years of the onset of severe symptoms
1Carabello
BA, et al. Lancet 2009; 373:956-66
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Treatment of Severe Aortic Stenosis
• Surgical aortic valve replacement (SAVR) is the gold standard
for treatment of severe AS
• However, 33% of all patients ≥75 years of age with severe AS
are declined for surgery1
• The mortality rate associated with SAVR increases substantially if certain
comorbidities are present
– Left ventricular dysfunction
– Previous cardiac operations
– Chronic obstructive pulmonary disease
– Liver or renal failure
– Frailty
1lung
B, et al. Eur Heart J. 2005; 26(24):2714-2720
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Treatment of Severe Aortic Stenosis
• Transcatheter aortic valve implantation (TAVI) is a viable treatment
strategy for AS patients at high or extreme surgical risk
• TAVI has demonstrated significant survival improvements compared to medical
management in inoperable patients in the PARTNER B trial1
• High risk patients receiving TAVI had similar survival rates to SAVR in the PARTNER A
trial2
PARTNER B
1Kapadia
PARTNER A
, et al., presented at TCT 2012 2Kodali S, et al., presented at ACC 2012
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
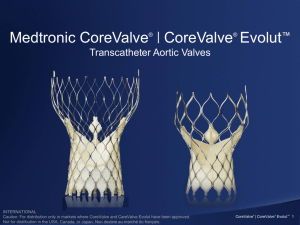
The Medtronic CoreValve System
• The Medtronic CoreValve® System has been implanted in more
than 45,000 patients in more than 60 countries worldwide.
•
•
•
•
•
Self-expanding nitinol frame
Tri-leaflet porcine pericardial tissue valve with supra-annular function
Four sizes (23, 26, 29, and 31 mm) to fit aortic annuli ranging from 18 to 29 mm
18-Fr delivery catheter for all valve sizes
Delivery from a transfemoral, subclavian, or direct aortic approach
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
CoreValve® System Clinical Experience
Medtronic CoreValve
Clinical Research Portfolio
Confirm Efficacy and
Optimize Practice
Expand Access to New
Populations and
Markets
Demonstrate Safety
and Efficacy
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
CoreValve® System Clinical Experience
Medtronic CoreValve
Clinical Research Portfolio
at end
of presentation
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc.References
2013. All Rights
Reserved.
Non destiné au marché français.
CoreValve® System Clinical Experience
National CoreValve Registries
References at end of presentation
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival
• When comparing all-cause survival across data sets, it is
important to remember that many variables can influence a
given rate.
• Baseline characteristics, patient selection practices, operator
experience, complication management techniques, and in
the case of registries, reporting practices, all play a role.
• Comparisons across data sets should be used to convey
general trends, not to derive specific conclusions regarding
TAVI devices.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ 30-day
National Registries and Medtronic Sponsored Studies
(independent studies, not head-to-head comparisons)
CoreValve
1Chiam,
et al., presented at EuroPCR 2013; 2Meredith, et al., presented at TCT 2012; 5Linke, et. al. presented at EuroPCR 2013 4Rueck, et al., presented at London Valves
2012; 5Tamburino, et al., Circulation 2011; 123: 299-308; 6Moat, et al. J Am Coll Cardiol 2011; 58(20): 2130-38; 7Godino, et al., J Am Coll Cardiol Intv 2010; 3: 1110-21;
8Avanzas, et al., Rev Esp Cardiol 2010; 63(2): 141-8; 9Bosmans, et al., ICVTS 2011; 12: 762-7; 10Gilard, et al. N Engl J Med 2012; 366: 1705-15
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ 1 Year
National Registries and Medtronic Sponsored Studies
(independent studies, not head-to-head comparisons)
CoreValve
1Meredith,
et al., presented at TCT 2012; 2Rueck, et al., presented at London Valves 2012; 3Tamburino, et al., Circulation 2011; 123: 299-308; 4Avanzas, et al., Rev Esp
Cardiol 2010; 63(2): 141-8; 5Linke, et. al. presented at EuroPCR 2013; 6Bosmans, et al., ICVTS 2011; 12: 762-7; 7Moat, et al. J Am Coll Cardiol 2011; 58(20): 2130-38;
8Gilard, et al. N Engl J Med 2012; 366: 1705-15
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ 1 Year
Rigorous Industry-Sponsored TAVI Studies
(independent studies, not head-to-head comparisons)
1Meredith,
5Leon,
et. al. presented at TCT 2012; 2Linke, et. al. presented at EuroPCR 2013; 3Leon, et. al. presented at ACC 2013; 4Smith, et al. N Engl J Med 2011; 364: 2187-98;
et al. N Engl J Med 2010; 363: 1597-1607
All routes
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ 1 Year
Select Contemporary Data Sets
(independent studies, not head-to-head comparisons)
1Meredith,
5Smith,
et. al. presented at TCT 2012; 2Linke, et. al. presented at EuroPCR 2013; 3Windecker, et. al. presented at EuroPCR 2013; 4Leon, et. al. presented at ACC 2013;
et al. N Engl J Med 2011; 364: 2187-98; 6Leon, et al. N Engl J Med 2010; 363: 1597-1607
All routes
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ 1 Year
National Registries and Post-Market Approval Studies
(independent studies, not head-to-head comparisons)
1Tamburino,
et al., Circulation 2011; 123: 299-308; 2Linke, et. al. presented at EuroPCR 2013; 3Windecker, et. al. presented at EuroPCR 2013; 4Moat, et al. J Am Coll
Cardiol 2011; 58(20): 2130-38; 5Thomas, et al., presented at TCT 2012; 6Gilard, et al. N Engl J Med 2012; 366: 1705-15
All routes
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
All-cause Survival │ Longer-Term
(independent studies, not head-to-head comparisons)
The Italian registry1 and the Canadian registry2 have reported
longer-term survival data on patient subsets
1Ussia,
et al., Eur Heart J 2012; 33: 969-976; 2Rodes-Cabau, et al. J Am Coll Cardiol 2012; 60(19): 1864-75
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Efficacy
• New York Heart Association (NYHA) Class status in the
ADVANCE study
• Health related quality of life (HRQoL) in the ADVANCE
study
• Hemodynamics
– 1-year CoreValve performance in the ADVANCE study
– 3-year CoreValve performance in the Italian Registry
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Efficacy │ New York Heart Association Class (NYHA)
The ADVANCE study demonstrated significant improvement in
functional status at 1 month post-TAVI, which was sustained to 1 year
1Linke
et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Efficacy │ Health Related Quality of Life
6-month Outcomes from the ADVANCE Study
The ADVANCE study demonstrated significant improvements in
post-TAVI quality of life using multiple measurement tools
1Bosmans,
et al., presented at TCT 2012
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Hemodynamics │ CoreValve ADVANCE Study
The ADVANCE study has demonstrated that the CoreValve EOA and
mean gradient remain stable out to 1 year
1Linke,
et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Hemodynamics │ Italian CoreValve Registry
The Italian Registry has demonstrated that the CoreValve EOA and
mean gradient remain stable out to 3 years
1Ussia,
et al., Eur Heart J 2012; 33: 969-76
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Safety
• Acute outcomes in the ADVANCE study
• Long term safety measures
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Safety │ Acute Outcomes
The ADVANCE Study
The ADVANCE study shows that procedural
complications are rare during CoreValve implants
1Linke,
et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Safety │ Long-Term Outcomes
Medtronic Foundational Studies
Two foundational CoreValve studies report no unexpected valve
malfunctions or structural deterioration out to 4 years after implant
a Change
in NYHA function resulting from intrinsic valve abnormality resulting in stenosis or regurgitation.
1Gerckens,
et al., presented at EuroPCR 2011; 2Gerckens, et al., presented at ESC 2011
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Procedural Success
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Procedural Success
National Registries and Medtronic Sponsored Studies
(independent studies, not head-to-head comparisons)
CoreValve
1Chiam,
et al., presented at EuroPCR 2013; 2Moat, et al. J Am Coll Cardiol 2011; 58(20): 2130-38; 3Meredith, et al., presented at TCT 2012; 4Avanzas, et al., Rev Esp Cardiol
2010; 63(2): 141-8; 5Bosmans, et al., ICVTS 2011; 12: 762-7; 6Gilard, et al. N Engl J Med 2012; 366: 1705-15; 7Linke, et. al. presented at EuroPCR 2013; 8Rueck, et al.,
presented at London Valves 2012; 9Tamburino, et al., Circulation 2011; 123: 299-308;
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications
• Definition
• Predictors
• Incidence
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Definition
• According to VARC 1, Major Vascular Complications include all complications
that can be caused by a wire and/or are related to vascular access, which
lead to death, need for significant blood transfusions, unplanned intervention,
or irreversible end-organ damage:
VARC 1 Definition1
• Ventricular perforation
• Aortic dissection or
rupture
• Iliofemoral dissection
or rupture
• Pseudoaneurysms
• Closure failure
• The VARC 2 definition2 downgraded unplanned intervention during the index
procedure to a minor vascular complication, except if it was associated with
qualifying consequences.
1Leon,
et al., J Am Coll Card 2011; 57 (3): 253-69; 2Kappetein, et al., J Am Coll Cardiol 2012; 60: 1438-54
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Predictors
Patient-Related
Factors
Anatomical Factors
• Femoral artery
calcium score2, 4
• Sheath to femoral
artery ratio (SFAR)2
• Female gender1
• Vessel tortuosity4
Procedural Factors
(Device / Operator)
• Learning curve2
• Complication
management3
• Percutaneous closure
device failure5
• Patient selection3
1Genereux,
et al. J Am Coll Cardiol 2012; 60(12): 1043-52; 2Hayashida, et al., J Am Coll Cardiol Cardiovasc Int 2011; 4(8): 851-8; 3Webb, et al., presented at TCT 2012;
et al. Cardiovasc Ther 2013; epub; 5Cockburn, et al., Cath and Cardiovasc Interv 2012; 79: 143-49
4Vavuranakis,
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Predictors
Effect of sheath size: the PRAGMATIC initiative showed patients treated with a
22F or 24F delivery sheath had significantly more vascular complications than
patients treated with smaller caliber devices
(green bar represents Sapien, yellow is a mixture of Sapien XT and CoreValve)
1Van
Mieghem, et al., Am J Cardiol 2012; 110(9): 1361-7
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Predictors
(independent studies, not head-to-head comparisons)
Effect of learning curve: four studies confirm that increased operator
experience decreases the incidence of major vascular complications
1Fearon,
et al., presented at ACC 2013; 2Hayashida, JACC Card Int 2011; 4(8): 851-8; 3Nuis, Am J Cardiol 2011; 107: 1824-1829; 4Toggweiler, JACC 2012; 59(2): 113-8
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Incidence
• Major vascular complication rate can be impacted by
variables unrelated to the TAVI device (e.g., patient selection
practices, operator experience and technique, complication
management strategy, percutaneous closure device function,
reporting practices in the case of registries).
• Examining outcomes over the wider clinical experience may
lessen the impact of any one of these variables and provide a
sense of TAVI device performance.
• However, it is important to be mindful that all of these
factors do contribute to the incidence of major vascular
complications and should be considered when doing
incidence comparisons across studies.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Incidence
Registry Data on CoreValve According to VARC 1
(independent studies, not head-to-head comparisons)
1Linke,
et al., presented at EuroPCR 2013; 2Gilard, et al. N Engl J Med 2012; 366: 1705-15; 3Meredith, et al., presented at TCT 2012; 4del Valle, et al., presented at London
Valves 2012; 5Ussia, et al., presented at EuroPCR 2012
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Major Vascular Complications │ Incidence
Select Data Sets Reporting According to VARC 1*
(independent studies, not head-to-head comparisons)
1Leon,
et al., presented at ACC 2013; 2Fearon, et al., presented at ACC 2013; 3Sack, et al., presented at TCT 2012; 4Linke, et al., presented at EuroPCR 2013; 5Treede, et al.,
presented at EuroPCR 2013; 6Gilard, et al. N Engl J Med 2012; 366: 1705-15; 7Meredith, et al., presented at TCT 2012; 8del Valle, et al., presented at London Valves 2012;
9Ussia, et al., presented at EuroPCR 2012
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation (AR)
• Definition
• Predictors
• Incidence
– Select Studies
– CoreValve ADVANCE Study Outcomes
• Clinical Impact
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Definition
• Aortic regurgitation (AR) can
be transvalvular (central),
paravalvular (PVL) or both.
Total AR = PVL + Transvalvular Leak
• What is clinically relevant is
the total volume of flow
through the valve
Image courtesy of Dr. J. Sinning, University of Bonn
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Predictors
Patient-Related
Factors
Anatomical Factors
• Calcified annulus
(commissures, cusps,
landing zone)3,4
• LVOT-AO angle1
• Male gender2,3
• Renal failure2
• Peripheral vascular
disease1
Procedural Factors
(Device / Operator)
• Large annulus2
• Transfemoral
approach9
• Elliptical annulus3
• Implant depth1,5,8
• LVOT diameter5
• Learning curve6
• Cover index (patientprosthesis
mismatch)4,5,6,7
• NYHA Class IV3
1
Sherif, et al., J Am Coll Cardiol 2010; 56: 1623-9 ; 2Abdel-Wahab, et al., Heart 2011; 97: 899-906; 3Unbehaun, et al., J Am Coll Cardiol 2012; 59: 211-21; 4Gripari, et al.,
Heart 2012; 98: 1229-1236; 5Italy: Petronio, et al. The Italian CoreValve Registry. TCT 2012; 6Detaint, et al., J Am Colll Cardiol Intv 2009; 8: 821-7; 7Santos, et al., Eur H J
Cardiovasc Img 2012; 13: 931-37; 8Chorianopoulos, et al., J Interven Cardiol 2012; 25: 174-179; 9Van Belle, et. al. Peri-valvular Aortic Regurgitation in Balloon-expendable
and Self-expendable TAVI procedures: Predictors and Impact on clinical outcome - Insights from the FRANCE2 Registry; TCT 2012
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Predictors
Effect of approach: FRANCE 21 and PARTNER2,3 demonstrate that the
transfemoral approach (TF) results in approximately 50% more total AR
than the transapical (TA) approach
1Van
Belle, et. al., presented at TCT 2012; 2Kodali, et al., presented at TCT 2012;3Dewey, et. al., presented at TCT 2012;
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Predictors
Effect of calcification: heavy calcification may prevent perfect
apposition to the native annulus, increasing the risk of PVL
Mechanism of PVL in calcified valves2
Images of increasing levels of valve
calcification1
1John,
et al., J Am Coll Cardiol Intv 2010; 3: 233-43; 2Sinning, et al, J Am Coll Cardiol 2012; 59: 1134-41
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Predictors
Effect of implant depth: appropriate implant depth minimizes potential flow
around the valve skirt. With CoreValve, a depth of 4-6 mm is currently
recommended to minimize paravalvular leak.
Mechanism of PVL in deep implant1
Mechanism of PVL in shallow implant1
1Sinning,
et al, J Am Coll Cardiol 2012; 59: 1134-41; 2Tchetche, et al., EuroIntervention 2012; epub
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
CoreValve ADVANCE
| Aortic Regurgitation
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
CoreValve ADVANCE
| Paravalvular Leak
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
CoreValve ADVANCE
| Transvalvular Regurgitation
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Incidence
• ASE / ESC guidelines, which are also recommended by VARC, are typically used
to grade the severity of AR using echo.
–
NOTE: Until VARC 2, there was no consensus for measuring paravalvular leak in transcatheter
valves.
• Standardized criteria can be open to interpretation. It is important to verify
that the severity grading criteria are the same when comparing AR across
studies.
–
Is “mild” AR included in the study-specific definition of “significant” AR?
• In addition to the semi-quantitative nature of AR grading, intraobserver
variability and variable baseline and procedural characteristics make it very
difficult to do a robust comparison of AR across studies.
• Medtronic- and Edwards-sponsored TAVI studies apply similar echo criteria to
grade the severity of AR, making it reasonable to compare the outcomes of
these studies.
• It is difficult to draw strong conclusions about a TAVI device by doing crossstudy comparisons.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation │ Incidence at 1 Year
Select Contemporary Data Sets
(independent studies, not head-to-head comparisons)
1Leon,
et. al. presented at ACC 2013; 2Linke, et. al. presented at EuroPCR 2013; 3Leon, et al. N Engl J Med 2010; 363: 1597-1607; 4Smith, et al. N Engl J Med 2011; 364:
2187-98; 5Windecker, et. al. presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Paravalvular Leak (PVL) │ Incidence at 1 Year
Select Contemporary Data Sets
(independent studies, not head-to-head comparisons)
1Leon,
et. al. presented at ACC 2013; 2Linke, et. al. presented at EuroPCR 2013; 3Leon, et al. N Engl J Med 2010; 363: 1597-1607; 4Smith, et al. N Engl J Med 2011; 364:
2187-98; 5Windecker, et. al. presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Paravalvular Leak (PVL) │ Incidence at 1 Year
Select Contemporary Data Sets—Transarterial Cohorts
(independent studies, not head-to-head comparisons)
1Leon,
et. al. presented at ACC 2013; 2Kodali, et al., presented at TCT 2012; 3Linke, et. al. presented at EuroPCR 2013; 4Leon, et al. N Engl J Med 2010; 363: 1597-1607;
et. al. presented at EuroPCR 2013
5Treede,
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Aortic Regurgitation* │ Clinical Impact
Medtronic CoreValve ADVANCE Study
*At discharge
1Linke,
et al. Presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke
• Predictors
• Timing
• Incidence
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Predictors
Risk factors of early stroke tend to implicate embolization of annular debris,
while risk factors of late stroke point to comorbid conditions.
Early Stroke
(within 30 days of TAVI)
Late Stroke
(30 days to 1 year after TAVI)
• Female gender1
• History of CABG1
• Surgical cutdown for access1
• History of stroke3,5
• New onset atrial fibrillation2
• Coronary artery disease3
• Baseline aortic regurgitation 3+2
• Peripheral vascular disease5
• Smaller aortic valve area3
• Baseline NYHA class3
• COPD4
• Chronic atrial fibrillation5
• BMI <25 kg/m4
• Multiple implant attempts4
• Valve dislodgement5
• Balloon post-dilatation5
1
Bosmans, et al., presented at EuroPCR 2013; 2Nuis, et al., Am J Cardiol 2012; 109: 1637-43; 3Miller, et al., J Thorac Cardiovasc Surg 2012;143(832-843): e13; 4Stortecky, et
al., EuroIntervention 2012; 8: 62-70; 5Nombela-Franco, et al., Circulation 2012; 126(25): 3041-53
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Type and Timing
The ADVANCE study showed that most strokes fall within 30 days of TAVI. However,
due to their multi-morbid conditions, TAVI patients remain at risk for stroke long after
their procedure.
30-day Stroke Rate in ADVANCE: 3.0%
1
Bosmans, et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Rate over Time
In the ADVANCE study, the overall stroke rate was low and
remained relatively stable over time
1
Bosmans, et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Learning Curve
Multicenter registry data on 4,563 patients indicate that CoreValve has a historically low
stroke rate, staying below 4.5% for any given cohort. It appears there is minimal learning
curve effect for this outcome.
Arrows indicate implant period and the reported rate of total stroke for the CoreValve cohort
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Incidence
• Stroke rate is strongly impacted by patient comorbidities,
and the timing of stroke events may indicate different
mechanisms at play.
• When doing a comparison of stroke rate across studies, it is
important to remember that the baseline patient
characteristics can vary greatly.
• It is very difficult to draw conclusions about the performance
of a particular TAVI device when comparing the incidence of
stroke rate across studies.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Stroke │ Incidence at 30 Days
Select Data Sets
(independent studies, not head-to-head comparisons)
1Leon,
et al. N Engl J Med 2010; 363: 1597-1607; 2Smith, et al. N Engl J Med 2011; 364: 2187-98; 3Thomas, et al., presented at TCT 2012; 4Leon, et al., presented at ACC
2013;
et al., presented at TCT 2012; 6Bosmans, et al., presented at EuroPCR 2013
5Meredith,
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation (PPI)
• Predictors
• Incidence
• Clinical Impact
– Post-TAVI PPI
– Post-TAVI Left Bundle Branch Block (LBBB)
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Predictors
More than 25 studies have been published on predictors of post-TAVI
conduction disturbances (CDs) and permanent pacemaker implant (PPI)
Patient-Related
Factors
Anatomical Factors
• Right Bundle Branch
Block (RBBB)2,3,4,5,7
• Variations in location of
LBBB exit point1
• Other pre-existing
conduction
disturbances3,4,8,9
• Septum
• Male
gender3
• Age > 75 years9
• Previous MI3
thickness1,6
• Thickness of the noncoronary cusp1
Procedural Factors
(Device / Operator)
• Implant Depth2,3,7
• Application of PPI
guidelines10
• Learning Curve11
• Balloon Aortic
Valvuloplasty8
• Radial force of the
prosthesis3
1Jilaihawi,
et al. Am Heart J 2009; 2Munoz-Garcıa, et. al. JACC CV 2012; 3Piazza et. al. EuroIntervention 2010; 4De Carlo , et. al. Am Heart J 2012; 5Calvi, et. al. JICE 2011;
et. al. Cath Card Intv 2012; 7Fraccarao, et. al. Am J Card 2011; 8Khawaja, et. al. Circ 2011; 9Schroeter et. al. EuroPACE 2011; 10Wenaweser, et. al. presented at
EuroPCR 2013; 11Meredith, et. al. presented at TCT 2012
6Saia,
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Predictors
Effect of implant depth: data from independent studies plotted
on the same graph show the strong relationship between PPI
rate and implant depth
1Tchetche,
2012;
et al. EuroIntervention 2012; 2Munoz-Garcıa, et. al. JACC CV 2012; 3Piazza et. al. EuroIntervention 2010; 4De Carlo , et. al. Am Heart J
et. al. JICE 2011; 6Saia, et. al. Cath Card Intv 2012; 7Fraccarao, et. al. Am J Card 2011; 8van der Boon, et. al. Int J Card 2013;
5Calvi,
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Predictors
Effect of learning curve: the rate of PPI in the CoreValve Australia-New
Zealand Study decreased over time as operators gained experience
Enrolling centers:
6
9
10
10
10
10
10
10
PPM rates in 6-mo blocks of pt enrollment, except most recent is 8-mo.
1Muller, et
al. Presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Predictors
Effect of PPI guideline use: varying 30-day pacemaker rates
across geographies in the ADVANCE study may reflect
differences in the application of PPI guidelines
Countries with less than 15 implants not shown
1Wenaweser,
et al. Presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Incidence
• The permanent pacemaker implantation rate can be
impacted by variables unrelated to the TAVI device (e.g.,
baseline conduction disturbances, anatomical differences,
clinician preference for prophylactic pacemaker use, and
operator experience).
• These factors should be kept in mind when comparing the
incidence of permanent pacemaker implantation across
studies.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implantation │ Incidence
CoreValve Outcomes in National Registries and Studies
(independent studies, not head-to-head comparisons)
1Chiam,
et al., presented at EuroPCR 2013; 2Tamburino, et al., Circulation 2011; 123: 299-308; 3Brito, et.al., presented at TCT 2011; 4Gilard, et al. N Engl J Med 2012; 366:
1705-15; 5Moat, et al., J Am Coll Cardiol 2011; 58(20): 2130-38; 6del Valle, et al., presented at London Valves 2012; 7Wenaweser, et al., presented at EuroPCR 2013;
8Muller, et al., presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Permanent Pacemaker Implant │ Clinical Impact
Both the ADVANCE study1 and the Australia-New Zealand Study2 showed
no impact of a new permanent pacemaker implant on mortality
1Wenaweser,
et al. Presented at EuroPCR 2013; 2Muller, et al. Presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Left Bundle Branch Block │ Clinical Impact
Current Research is Inconclusive Regarding Mortality Impact of LBBB Post –TAVI
LBBB Post-TAVI
Mortality Impact
Patients not receiving permanent
pacemaker before discharge.
LBBB Post-TAVI
NO Mortality Impact
De Carlo, et. al. AHJ 2012, (N=275)
Houthuizen, et. al. Circulation 2012, (N=679)
Urena, et. al. JACC 2012, (N=202)
Muller, et. al. EuroPCR 2013, (N=200)
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Left Bundle Branch Block │ Clinical Impact
Both the ADVANCE study1 and PARTNER2 showed no impact of a
new LBBB on mortality
1Wenaweser,
et al. Presented at EuroPCR 2013; 2Nazif, et al. Presented at ACC 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
Left Bundle Branch Block │ Clinical Impact
Both the ADVANCE study1 and the Australia-New Zealand Study2
showed no significant adverse effect of a new LBBB at discharge on
the mean LV ejection fraction
1Wenaweser,
et al. Presented at EuroPCR 2013; 2Muller, et al. Presented at EuroPCR 2013
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.
References for slides 8, 9, and 54
1. ClinicalTrials.gov Identifier: NCT01240902
2. Buellesfeld L, et al. J Am Coll Cardiol 2011; 57: 1650-7.
3. ClinicalTrials.gov Identifier: NCT01586910
4. ClinicalTrials.gov Identifier: NCT01675440
5. Meredith IT, et al. Presented at TCT 2012
6. Medtronic CoreValve Japan Trial, protocol MDT-2111
7. ClinicalTrials.gov Identifier: NCT01531374
8. Linke A, et al. Presented at EuroPCR 2013.
9. ClinicalTrials.gov Identifier: NCT01624870
10. ClinicalTrials.gov Identifier: NCT01676727
11. France II Registry. Eltchaninoff H, Presented at TVT 2013
12. Italian Registry. Ussia GP, et al. Presented at EuroPCR 2012
13. Italian Registry. Petronio AS, et al., Presented at TCT 2012.
14. Israel Registry. Kornowski R, et al. Presented at EuroPCR 2012.
15. UK Registry. Moat NE, et al. J Am Coll Cardiol 2011; 58: 2130-8.
16. UK Registry. Moat NE, et al. Presented at TCT 2012.
17. Belgian Registry. Bosmans J, et al. Presented at EuroPCR 2012
18. Bosmans J, et al., Interact Cardiovasc Thorac Surg 2011; 12(5): 762-7.
19. Brazilian Registry. Brito FS, et al. Presented at TCT 2011.
20. Spanish Registry. Avanzas P, et al., Rev Esp Cardiol. 2010;63:141-8.
21. Milan Registry. Buchanan GL, et al. Presented at EuroPCR 2012.
22. Asia Registry. Chiam P, Presented at EuroPCR 2013.
23. del Valle R, et al., Presented at PCR London Valves 2012
24. Ruck A, et al., Presented at PCR London Valves 2012
25. Figulla H, et al., Presented at TCT 2012
26. GARY, Mohr F, Presented at ACC 2013
27. German TAVI Registry: Sinning, et al., Am Heart J 2012; 164: 102-110.e1
CoreValve® is a registered trademark of Medtronic CV Luxembourg S.a.r.l.
INTERNATIONAL. CAUTION—For distribution only in markets where CoreValve has been approved. Not approved in the USA or Japan. ©Medtronic, Inc. 2013. All Rights Reserved.
Non destiné au marché français.