Protecting Intellectual Property in a Competitive Market
advertisement

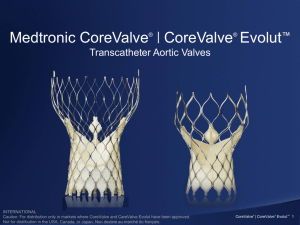
Protecting Intellectual Property in a Competitive Market Sabing Lee Andrew Douglas Partner Knobbe Martens Olsen & Bear Partner Knobbe Martens Olsen & Bear Presented by: Protecting Intellectual Property in a Competitive Market - Recent IP News You Need to Know Andrew Douglas and Sabing Lee October 22, 2014 MDIF 2014 Overview • Medical device patent statistics • Patentable subject matter • Non-practicing entity (NPE) litigation • Challenging patents at the PTO • Important litigation – Masimo v. Philips – Edwards v. Medtronic • Audience questions Medical Device Patent Statistics Applications Filed and Patents Granted Per Year *data from USPTO website (as of December 31, 2013) Medical Device Patents Granted *data from USPTO website (as of December 31, 2013) 2013 Medical Device Patent Owners TOP TEN (tie) *data from USPTO website (as of December 31, 2013) Patentable Subject Matter Limits of What Can Be Patented • §101 – Inventions Patentable • Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefor ... • In 1980, the Supreme Court said a bacterium capable of breaking down crude oil could be patented • Intent of Congress to allow patents be issued to “anything under the sun that is made by man” • In 1998, the Federal Circuit said that business methods and software could be patented • Must produce a “useful, concrete and tangible result” Alice v. CLS Bank – The Technology • Claims to a computerized invention to mitigate “settlement risk” • Risk that only one party to an agreed-upon financial exchange will satisfy its obligation. • Use a third-party intermediary to create “shadow records” that track and control currency trades between banks. • CLS is an international bank consortium that clears $5B in trades every day. Exceptions to Patentable Subject Matter • §101 – Inventions Patentable • Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefor .. . • Implicit exception to§101: • laws of nature, natural phenomena, and abstract ideas • Supreme Court explains that it is concerned about pre-emption • Pre-empting use of abstract idea of hedging risk (Bilski) • Laws of nature, NPs and AIs are “the basic tools of scientific and technological work.” (Myriad) • Don’t want to improperly tie up the future use of these building blocks of human ingenuity. (Mayo, citing Morse) What May Now Be Hard To Patent • Methods that can be : • Found in the prior art, performed on a computer • Performed in the mind • Performed with pencil and paper • Performed between two individuals [traditional business transactions] • Methods that recite : • Laws of nature, with insignificant pre-solution activity [data gathering] • Natural phenomena, with insignificant post-solution activity [display result] Non-Practicing Entity (NPE) Litigation What Rights Does A Utility Patent Confer? • Confers right to: • Exclude others from making, using and selling claimed invention • For 20 years from earliest claimed filing date • Does not give patent owner right to make, use or sell invention • There is no requirement for the patent owner to make a commercial product in order to enforce its patent What Is A Non-Practicing Entity (NPE)? • • • • • • Universities Individual inventors Research & development firms Operating companies (OC) that sue non-competitors Companies that file for patents but do not sell products Patent monetization entities (PME) or patent-assertion entities (PAE) – Companies that purchase patents to assert them – Companies that used to produce products NPE Litigation Statistics • 3,608 cases filed in 2013 • 67% of all patent litigation cases in 2013 were filed by NPEs • 91% of all NPE litigations were brought by patent assertion entities Source: RPX Corp. • 745 unique operating healthcare/pharma defendants in NPE litigation Source: PatentFreedom (as of July 14, 2014) What Happened In Congress? • • • Innovation Act, H.R. 3309 – Intended to curb abusive litigation practices – Specific identification of patent claims and allegedly infringing products – Limit discovery to reduce litigation cost – Awarding of attorneys’ fees to the prevailing party Passed by House 325-91 in December 2013 Removed from Senate in May 2014 LifePort/LifeScreen Litigation • September 2012 - Acacia Research (known NPE) enters into settlement agreement with Boston Scientific • December 2012/January 2013 - Acacia Research acquires patents from Boston Scientific relating to vena cava filters and endovascular grafts • 2012-2014 - LifePort Sciences LLC and LifeScreen Sciences LLC, affiliated with Acacia, sue Cook, Endologix, Medtronic, C.R. Bard, Cordis and W.L. Gore asserting infringement of vena cava filter and endovascular graft patents Orthophoenix Litigation • 2007 - Medtronic acquires spinal device maker Kyphon for ~$4 billion • April 2013 - Medtronic sells over 500 patents to Orthophoenix, LLC, an entity associated with IP Nav, a known NPE. • June-October 2013 – Orthophoenix sues 7 companies (Wright Medical, DFine, Osseon, Ascendx, Sintea Plustek, Soteira/Globus, and Stryker) Kardiametrics Litigation • October 2000 – Medtronic acquires PercuSurge, maker of embolic protection devices for $225 Million • April 2013 – Kardiametrics acquires patents from Medtronic • September 20, 2013 – Kardiametrics sues Abbott, Boston Scientific, Control Medical Technology, Cordis, Covidien, Medrad and Merit Medical Systems Bonutti Litigation • More than 250 patents developed by Dr. Peter Bonutti, an orthopedic surgeon • Dr. Bonutti partnered with Acacia • Lawsuits against MicroPort Orthopedics, Lantz Medical, Zimmer, Wright Medical, Arthrex, DePuy, ConforMIS, Smith & Nephew, Linvatec, Biomet Challenging Patents at the PTO How Do You Challenge An Issued Patent? In Court • Seek declaratory judgment of invalidity At the USPTO (after AIA) • Ex Parte Reexam • Post-Grant Review (PGR) (for applications filed after March 16, 2013) • Inter Partes Review (IPR) • Covered Business Method (CBM) (for financial products and services) • Derivation Proceedings Summary of Potential Third-Party Involvement Filing Issue 9 mo. Post-grant review (PGR) ($$$) Inter partes review (IPR) ($$$) Derivation action/proceeding ($$$) Pre-issuance Submission ($) Ex parte reexamination ($$) IPR Final Written Decisions IPR and CBM Filings by Technology Medical Device IPR Overview • KnobbeMedical.com identified 70 IPRs relating to medical devices that had activity between August 2013 & August 2014 (65 in litigation) • Top filers – Medtronic, Smith & Nephew, Boston Scientific, Zimmer, Wright Medical • Top defendants – Acacia (Bonutti Skeletal, Endotach, LifePort Innovations), NuVasive NPE/PAEs Involved Masimo v. Philips Masimo v. Philips (Delaware) • Irvine-based Masimo Corporation alleged infringement of a family of patents directed to “pulse oximetry” technology that can provide accurate measurements in the presence of patient motion Monitor Cable Sensor Masimo v. Philips (Delaware) – Trial • In a first trial, Masimo asserted two patents • Shortly before trial, Philips admitted infringement. Philips challenged validity and the amount of damages. Masimo v. Philips (Delaware) – Trial • Philips asserted ‘074 Patent directed to “fuzzy logic” in a quality indicator Masimo v. Philips (Delaware) – Verdict • Jury found Masimo’s patents valid and awarded damages Masimo v. Philips (Delaware) – Verdict • Jury found that Masimo did not infringe Philips’ “fuzzy logic” patent Edwards v. Medtronic EW v. MDT/CoreValve – The Products Medtronic CoreValve Edwards Sapien EW v. MDT/CoreValve - World-Wide Fight Year Patent Country Plaintiff Defendant Outcome 2009 Andersen UK Edwards CoreValve Not Infringed Affirmed on Appeal 2008 Andersen Germany (Infrin’t) Edwards CoreValve Not Infringed Affirmed on Appeal 2010 Andersen Germany (Invalidity) CoreValve Edwards Not Invalid 2010 Andersen Delaware Edwards CoreValve Infringed - $83M Affirmed-in-part 2012 Seguin California MDT Edwards Invalid 2013 Spenser Germany (Infrin’t) Edwards MDT Infringed (injunction) 2014 Spenser EPO (Invalidity) Edwards MDT Invalid 2014 Cribier California Edwards MDT Infringed - $392M EW v. MDT/CoreValve - World-Wide Fight Year Patent Country Plaintiff Defendant Outcome 2009 Andersen UK Edwards CoreValve Not Infringed Affirmed on Appeal 2008 Andersen Germany (Infrin’t) Edwards CoreValve Not Infringed Affirmed on Appeal 2010 Andersen Germany (Invalidity) CoreValve Edwards Not Invalid 2010 Andersen Delaware Edwards CoreValve Infringed - $83M Affirmed-in-part 2012 Seguin California MDT Edwards Invalid 2013 Spenser Germany (Infrin’t) Edwards MDT Infringed (injunction) 2014 Spenser EPO (Invalidity) Edwards MDT Invalid 2014 Cribier California Edwards MDT Infringed - $392M 2014 Andersen Delaware Edwards CoreValve Prelim. Injunction EW (Andersen) v. CoreValve - PI • Although the court acknowledged that the public interest would be well served by permitting CoreValve Generation 3 devices to be sold, the court noted that “[e]nforcing patent rights is especially important where there is egregious conduct to be addressed and deterred.” • The court held that the preliminary injunction must be tailored to permit Medtronic to sell Core Valve Generation 3 devices to patients that Edwards concedes cannot be served by Edwards' devices. EW v. MDT/CoreValve - Settlement MDT will pay EW: •A one-time payment of $750 million •Royalties through April 2022, not less than $40 million annually The parties agreed to: •Dismiss all of the pending litigation matters and patent office actions between them •Grant each other broad releases to patent litigation claims •Not sue each other “for patent matters anywhere in the world for eight years in the field of aortic and all other transcatheter heart valves.” Questions & Answers Questions • With all the cyber hacking occurring, what are the risks of sending IP info to lawyers and others via email, or should we use some other method? • What are the main differences with regard to patentable subject matter for medical devices in the US vs Europe? Questions • For a small medical device company in the start-up mode (nor probably anyone else, for that matter), it is not financially possible to file patents in every country OUS. What countries would be recommended for international patent filings? • For example, following the US, might one consider Europe, Japan, China, India? What about Canada and Australia or other Englishspeaking countries? • After the European phase, how do you decide which countries in Europe to approach? • How do you develop the right strategy for a small start-up against large corporations without spending a lot of capital on lawyers? Thank you! • andrew.douglas@knobbe.com • (949) 721-7623 sabing.lee@knobbe.com (949) 721-6360 Thank you