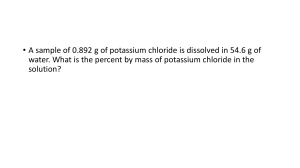Molarity and Molality Worksheet - Chemistry Problems
advertisement

CP CHEMISTRY Molarity and Molality WS Name: __________________________________ ____ period I. Molarity Problems Be sure to show all your work!! 1. What is the molarity of an aqueous solution that contains 14.2 g NaCl dissolved in 2.35 L of solution? 2. How many grams of NaCl would be contained in 1.00 L of the solution in #1? 3. A solution is made by dissolving 17.0 g of lithium iodide (LiI) in enough water to make 387 mL of solution. What is the molarity of the solution? 4. How many grams are contained in 150 mL of a 0.20 M solution of K2SO4? 5. Calculate the molarity of a water solution of CaCl2, given that 5.04 L of the solution contains 612 g of CaCl2. 6. Suppose you wish to make 0.85 L of a 0.25 M solution of silver nitrate, AgNO3? How many grams of the AgNO3 would you need to prepare the solution? CP CHEMISTRY, Molarity and Molality WS, page 2 II. Molality Be sure to show all work!!! 1. What is the molality of a solution that contains 16.3 g of potassium chloride, KCl, dissolved in 845 g of water? 2. What is the molality of a solution formed by mixing 104 g of silver nitrate, AgNO3, with 1.75 kg of water? 3. Suppose that 5.25 g of sulfur, S8, is dissolved in 682 g of the liquid solvent carbon disulfide, CS2. What is the molality of the sulfur solution? 4. What is the molality of a solution that contains 25 g of NaCl in 2.2 kg of water?