File
advertisement

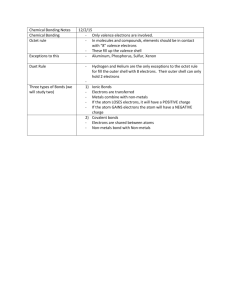
Unit 3 The Periodic Table & Chemical Bonding The elements of the periodic table can be divided into three main categories: Metals, Non-Metals, and Metalloids. . .. . . . . . Properties of Metals Found on the left side of the Periodic Table Metals are good conductors of heat and electricity. shiny. ductile (can be stretched into thin wires). malleable (can be pounded into thin sheets). A chemical property of metal is its reaction with water which results in rust/corrosion. Properties of Non-Metals Found on the right side of the Periodic Table Non-metals are poor conductors of heat and electricity. not ductile or malleable. Solid non-metals are brittle and break easily. They are dull. Many non-metals are gases. Sulfur Properties of Metalloids Located between Metals Silicon and Non-Metals on the PT Metalloids (metal-like) have properties of both metals and non-metals. They are solids that can be shiny or dull. ductile and malleable. conduct heat and electricity better than non-metals but not as well as metals. Periods Each horizontal row of elements is called a period. The elements in a period are not alike in properties. Each element in the same period has the same number of energy levels (electron shells) The first element in a period is always an extremelyactive solid. The last element in a period, is always an inactive gas. As you travel right across a period, you increase the number of protons in the nucleus by 1 Families Columns of elementsare called groups or families. Elements in each family have very similar properties. All elements in a family have the same number of valence electrons. As you move down a family, each new element has an extra energy level (electron shell) Hydrogen The hydrogen square sits atop Family IA, but it is not a member of that family. Hydrogen is in a class of its own. It is NOT in the next family. It’s a gas at room temperature. It has one proton and one electron in its one and only energy level. Hydrogen only needs 2 electrons to fill up its valence shell. Periodic Table Family Names Valence Electrons Each family has a set number of valence electrons. This is why each element in one family reacts similarly. 1 = Alkali Metals 2 = Alkali Earth Metals 3 = Boron Family 4 = Carbon Family 5 = Nitrogen Family 6 = Oxygen Family 7 = Halogen Family 8 = Nobel Gas Family Variable = Transition Metals Each family also has a specific OXIDATION NUMBER (More on Oxidation Numbers later) Notes on Specific Families Group 1: Alkali Metals Hydrogen is not a member, it is a non-metal 1 electron in the outer shell Often react with Halogen Family Very reactive, esp. with water Group 2: Alkaline Earth Metals 2 electrons in the outer shell Often reactive with Oxygen Family (less reactive than Alkali metals though) Notes on Specific Families Group 3-12: Transition Metals (That block in the middle) Valence electrons (and thus Oxidation Numbers) can vary For instance Cu can have 1, 2, 3, or 4 valence electrons depending on the version of Cu Good conductors of heat and electricity. Used for wiring, tools, jewelry, decorative metalwork, etc… Notes on Specific Families Group 13: Boron Family Most are metals Boron is a metalloid Group 14 & 15: Carbon Family AND Nitrogen Family Contains metals, metalloids, and a non-metal Contains metals, metalloids, and non-metals Families Notes on Specific Families Group 16: Oxygen Family Contains metals, metalloids, and non-metals Reactive: Generally with Alkaline Earth Metals Group 17: Halogen Gases All are non-metals Very reactive Often bonded with elements from Group 1 Notes on Specific Families Group 18: Noble Gas Family Exist as gases All are non-metals Not reactive with other elements because their valence shell is full 8 electrons in the outer shell = Full Helium (He) has only 2 electrons in the outer shell to be full Rare Earth Metals Lanthanide Series Actinide Series Some are Radioactive The rare earths are silver, silvery-white, or gray metals. Conduct electricity Actinides are found primarily in applications where their radioactivity can be used to power devices such as cardiac pacemakers. Recap! “Happy” Elements All elements want to be “happy” An atom wants a stable outer energy level to make them “happy” How do they become happy? Atoms will gain or lose electrons in order to become stable. Usually the most stable configuration involves having 8 valence e 8 electrons = full octet Octet Rule Atoms will form bonds with other atoms in order to have eight valence electrons. Can share or transfer electrons with other elements Elements will not just lose or gain electrons. They will give to another element or take from another Oxidation Numbers The oxidation number indicates how many electrons are going to be lost or gained during bonding. Range from -4 to +4 Predicting Oxidation Numbers 1. First, determine how many valence electrons the neutral atom has. 2. Determine if the atom will lose or gain electrons. Ask yourself, which would be faster? A) Losing down to zero B) Gaining up to eight 3.Count the number of electrons gained or lost. If you lose electrons, the charge will be (+) If you gain extra electrons, the charge will be (-) Practice What would the oxidation number be for the following atoms? A) Lithium 1+ B) Sulfur 2- C) Aluminum 3+ D) Iodine 1- E) Phosphorous 3 G) Neon 0 F) Carbon 4± Oxidation #s on the PT Chemical Stability Knowing this information about oxidation numbers, which elements are the most chemically stable elements? Noble gases - Are the most stable elements - They have 8 valence electrons and don’t want to gain or lose any electrons Chemical Bonds Only involve ELECTRONS The nucleus does not matter here Elements will not just lose or gain electrons. They will give to another element or take from another element There will always be AT LEAST 2 atoms involved in this process. Binary Compound = Compound with ONLY 2 elements in it NaCl, CaCl2, K2S, etc.. Types of Chemical Bonds There are 3 different ways that two (or more) atoms can interact or BOND This bonding will always end in a new COMPOUND with new chemical properties 3 types of bonds: Ionic Covalent Metallic Ionic Bonds Formed when one atom gains an electron and another atom loses an electron. One atom transfers their electrons to the other atom. Ionic Bonds Formed when one atom gains an electron and another atom loses an electrons. One atom transfers their electrons to the other atom. More on Ionic Bonds Occurs between a metal and a nonmetal. Two oppositely charged ions. The product is a neutral compound – and the oxidation #’s add to zero! If they don’t add to zero, you did it wrong. Properties of Ionic Compounds Stronger bonds High melting points Conduct electricity when in solution or in a molten state Generally dissolve in water Generally crystalline solid at room temp. Drawing Ionic Bonds Lets use NaCl (a metal and a nonmetal) 1st – Determine the oxidation numbers Na = 1+ Cl = 1 2nd – Draw the Lewis Structure for each atom 3rd – Draw the electron(s) being transferred in the proper direction DOUBLE CHECK: Does each atom have a full valence orbital? Exploring Ionic Bonds Remember that when atoms bond, they form new compounds with different properties. Types of Chemical Bonds There are 3 different ways that two (or more) atoms can interact or BOND This bonding will always end in a new COMPOUND with new chemical properties 3 types of bonds: Ionic Covalent Metallic Covalent Bonds Occur between two or more nonmetals. Formed when two atoms share electrons with one another. Can be between two different atoms, or between two atoms of the same element Also called Molecular compounds aka MOLECULE Properties of Covalent Bonds Weaker bonds Low melting and boiling points Do NOT conduct electricity when in solution Generally don’t dissolve in water. Generally gases or liquids at room temperature Drawing Covalent Bonds Lets use HCl (2 non-metals) 1st – Draw the Lewis Structure for each atom 2nd – Draw the electron(s) being shared 3rd – Draw each covalent bond as a line between the two atoms DOUBLE CHECK: Does each atom have a full valence orbital? PRACTICE COVALENT BONDS H2O CH4 CO2 Diatomic Molecules Formed between two atoms of the same element covalently bonded together. They cannot exist as lone atoms and must bond to something. Called Molecules H2 O2 N2 I2 Br2 Cl2 F2 forms a seven on the periodic table How do you know which type of bond is formed? IONIC - Metal and Nonmetal - Transfer Electrons COVALENT - Two Non Metals - Share Electrons Bond Types (cont’d) Metallic—Between two metals All metals want to give away electrons because they have + oxidation numbers Don’t transfer electrons specifically Allow electrons to f low from one atom to another. Can pass between many atoms. Writing and Naming Chemical Formulas Ionic vs. Covalent