Lighting a Bunsen Burner Lab
advertisement

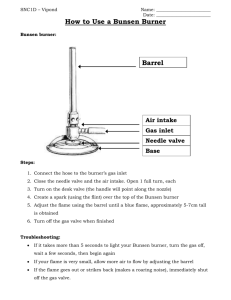
Section 1: Introduction to Chemistry and Lab Safety [LIGHTING A BUNSEN BURNER LAB] Purpose In this lab you will become familiar with the parts of a Bunsen burner (Tirrill burner). You will learn how to adjust the height and color of the flame. By the end of the lab you should understand the difference between complete and incomplete combustion. Introduction The Bunsen burner (or Tirrill burner) that you will be using in this class looks like the one shown. Procedure Get a burner out of the cabinet. Examine it and look at the figure above the right to identify all the parts of your burner. Make sure you have a turn manipulating the burner so you become familiar with how it operates. Twist the needle valve (the bottom screw) to close it completely. This prevents gas from flowing into the burner. Reopen it about halfway. Twist the barrel in the clockwise direction to close the air holes completely. Reopen it about halfway. Make sure your burner tubing is securely connected to the inlet at the base of the burner. This is where the gas enters the burner. Now connect the other end of the burner tubing to the gas nozzle at your lab station. Clear off your lab space of anything other than your equipment, notebook, and pencil. Try to light the burner. Turn the gas valve at the lab station to the “on” position (the valve will be parallel to the nozzle.). Quickly produce a spark with the striker over the top of the barrel of the burner. IF IT DOES NOT LIGHT WITHIN 20 SECONDS TURN OFF THE GAS. Fan the air around the burner and try again. You may wish to practice with the striker beforehand. If your burner doesn’t light, listen and make sure there is gas coming out of the burner before trying to light it a second time. You should hear the gas coming out. Now that the burner is lit, we are going to test out the different flames. Lower the barrel until the air intake openings are mostly closed. The resulting flame is a luminous flame. Observe and record the color of the luminous flame. Luminous flames are caused by the incomplete combustion of the fuel. Using crucible tongs, hold a clean, dry evaporating dish near the top of the luminous flame with the open side up until a deposit forms on the dish. Record the color of the deposit and speculate regarding the composition of the deposit. Allow the evaporating dish to cool, but leave the deposit on the bottom. Optimize your air intake openings to obtain the quiet, non-luminous flame making the flame as colorless as possible. Using crucible tongs, hold the evaporating dish in the flame just above the inner cone. What happens to the deposit on the dish? Explore the nature and temperature of different parts of the burner flame by holding a wood splint in the center of the flame at each of the following positions: resting on the top of the barrel, below the tip or top of the inner cone, just above the tip of the inner cone, and just above the top of the outer cone. You should only scorch the wood splint, do not allow it to burn and hide the original scorch pattern. Use a fresh portion of the wood splint or a different splint at each flame position. Either insert a picture or make a sketch in your lab notebook of each different scorch pattern. Also, suggest reasons why there are differences. Post-Lab Questions 1. How does adjusting the needle valve and barrel on the Bunsen burner affect the flame? 2. What happens to the flame if the air holes are opened too much? 3. What appeared on the bottom of the evaporating dish after it was heated? Where did it come from? 4. Using any resources that you need to use, define complete combustion and write a chemical equation for the complete combustion of methane gas. 5. Using any resources that you need to use, define incomplete combustion and write a chemical equation for the incomplete combustion of methane gas. 6. Why is the non-luminous (blue) flame preferred over the luminous (yellow) flame in the laboratory?