Patient Decisions for Disclosure of Secondary
advertisement
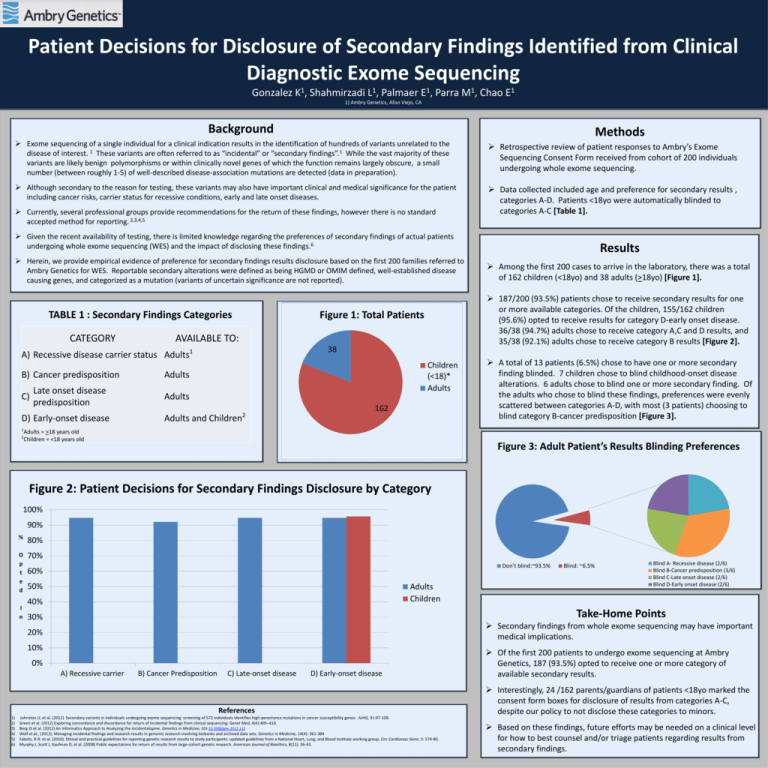
Patient Decisions for Disclosure of Secondary Findings Identified from Clinical Diagnostic Exome Sequencing Gonzalez 1 K, Shahmirzadi 1 L, Palmaer 1 E, Parra 1 M, Chao 1 E 1) Ambry Genetics, Aliso Viejo, CA Background Methods Exome sequencing of a single individual for a clinical indication results in the identification of hundreds of variants unrelated to the disease of interest. 1 These variants are often referred to as “incidental” or “secondary findings”.1 While the vast majority of these variants are likely benign polymorphisms or within clinically novel genes of which the function remains largely obscure, a small number (between roughly 1-5) of well-described disease-association mutations are detected (data in preparation). Retrospective review of patient responses to Ambry’s Exome Sequencing Consent Form received from cohort of 200 individuals undergoing whole exome sequencing. Although secondary to the reason for testing, these variants may also have important clinical and medical significance for the patient including cancer risks, carrier status for recessive conditions, early and late onset diseases. Data collected included age and preference for secondary results , categories A-D. Patients <18yo were automatically blinded to categories A-C [Table 1]. Currently, several professional groups provide recommendations for the return of these findings, however there is no standard accepted method for reporting. 2,3,4,5 Given the recent availability of testing, there is limited knowledge regarding the preferences of secondary findings of actual patients undergoing whole exome sequencing (WES) and the impact of disclosing these findings.6 Herein, we provide empirical evidence of preference for secondary findings results disclosure based on the first 200 families referred to Ambry Genetics for WES. Reportable secondary alterations were defined as being HGMD or OMIM defined, well-established disease causing genes, and categorized as a mutation (variants of uncertain significance are not reported). TABLE 1 : Secondary Findings Categories CATEGORY AVAILABLE TO: A) Recessive disease carrier status Adults B) Cancer predisposition Adults Late onset disease C) predisposition Adults D) Early-onset disease 38 Children (<18)* Adults Adults and Children 2 Among the first 200 cases to arrive in the laboratory, there was a total of 162 children (<18yo) and 38 adults (>18yo) [Figure 1]. 187/200 (93.5%) patients chose to receive secondary results for one or more available categories. Of the children, 155/162 children (95.6%) opted to receive results for category D-early onset disease. 36/38 (94.7%) adults chose to receive category A,C and D results, and 35/38 (92.1%) adults chose to receive category B results [Figure 2]. Figure 1: Total Patients 1 Results 162 A total of 13 patients (6.5%) chose to have one or more secondary finding blinded. 7 children chose to blind childhood-onset disease alterations. 6 adults chose to blind one or more secondary finding. Of the adults who chose to blind these findings, preferences were evenly scattered between categories A-D, with most (3 patients) choosing to blind category B-cancer predisposition [Figure 3]. 1 Adults = >18 years old 2 Children = <18 years old Figure 3: Adult Patient’s Results Blinding Preferences Figure 2: Patient Decisions for Secondary Findings Disclosure by Category 100% 90% % 80% O p t e d 70% I n Don’t blind:~93.5% 60% 50% Adults Children 40% Blind A- Recessive disease (2/6) Blind B-Cancer predisposition (3/6) Blind C-Late onset disease (2/6) Blind D-Early onset disease (2/6) Take-Home Points 30% Secondary findings from whole exome sequencing may have important medical implications. 20% 10% 0% A) Recessive carrier B) Cancer Predisposition C) Late-onset disease D) Early-onset disease References 1) 2) 3) 4) 5) 6) Blind: ~6.5% Johnston JJ, et al. (2012) Secondary variants in individuals undergoing exome sequencing: screening of 572 individuals identifies high-penetrance mutations in cancer-susceptibility genes. AJHG, 91:97-108. Green et al. (2012) Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genet Med, 4(4):405–410. Berg JS et al. (2012) An Informatics Approach to Analyzing the Incidentalopme. Genetics in Medicine, DOI:10.1038/gim.2012.112 Wolf et al., (2012). Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in Medicine, 14(4): 361-384 Fabsitz, R.R. et al. (2010). Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Gene, 3: 574-80. Murphy J, Scott J, Kaufman D, et al. (2008) Public expectations for return of results from large-cohort genetic research. American Journal of Bioethics, 8(11): 36-43. Of the first 200 patients to undergo exome sequencing at Ambry Genetics, 187 (93.5%) opted to receive one or more category of available secondary results. Interestingly, 24 /162 parents/guardians of patients <18yo marked the consent form boxes for disclosure of results from categories A-C, despite our policy to not disclose these categories to minors. Based on these findings, future efforts may be needed on a clinical level for how to best counsel and/or triage patients regarding results from secondary findings.









