Heat Treatment of Steels
advertisement

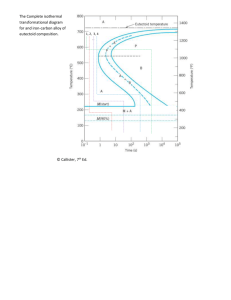
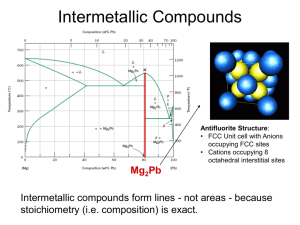
Phase Diagram MSE 201 Lab III Overview Examine microstructures of the selected carbon steels Correlate chemical composition and temperature with microstructure of the steel samples using equilibrium Fe-C phase diagram Hardness testing of the steels of equilibrium and non-equilibrium cooling Samples Conditions AISI-SAE 1018 – 0.18 % C AISI-SAE 1045 – 0.45 % C AISI-SAE 1095 – 0.95 % C Austenitized at 870°C for 2 hours followed by Slow cooling (furnace cool) Fast cooling (water quench) Iron-Carbon Phase Diagram: It’s All Greek To Me Alpha “Ferrite”, BCC Iron Room Temperature Gamma “Austenite”, FCC Iron Elevated Temperatures These are PHASES of iron. Adding carbon changes the phase transformation temperature. Where Does the Carbon Go? Interstitial Sites: FCC (Austenite) BCC (Ferrite) BCT (Martensite) Solubility Limits BCC ( or Ferrite) Iron can’t hold much Carbon, it has a low solubility limit (0.022%) But, FCC ( or Austenite) Iron can hold up to 2.14% Carbon! P 2.14 E 4.30 L + Fe3C F G x M O N H 0.76 0.022 Cementite Fe3C C x’ 6.70 Eutectoid Reaction (Pearlite Formation) Austenite precipitates Fe3C at Eutectoid Transformation Temperature (727°C). Cooling + Fe3C Heating When cooled slowly, forms Pearlite, which is a microcontituent made of ferrite () and Cementite (Fe3C), looks like Mother of Pearl. Microstructure of Pearlite Photomicrographs of (a) coarse pearlite and (b) fine pearlite. 3000X Hypo-Eutectoid “Proeutectoid” means it formed ABOVE or BEFORE the Eutectoid Temperature! Microstructure of Hypo-Eutectoid Hyper-Eutectoid “Proeutectoid” means it formed ABOVE or BEFORE the Eutectoid Temperature! Microstructure of Hyper-Eutectoid Exercise with Lever Rule • Figure out what type of reaction of the transformation and the phases/microconstituents involved • Construct a tie line at the temperature of alloy • Project the intersections to determine % concentration • C0 = C f + (1- f ) C Liq Exercise with Lever Rule – Hypo-eutectoid C0 = 0.1% C 1. Phase(s) present: , Fe3C 2. % C: - 0.022%, Fe3C – 6.7% 3. Amount of each phase: f - fraction of Then C0 = C f + (1- f ) C Fe3C f = (C0 - C Fe3C)/(C - C Fe3C) f Fe3C = 1- f 4. Microconstituents: Primary Ferrite, Pearlite 5. % C in each microconstituent: Ferrite - 0.022% Pearlite – 0.76% 6. Amount of each microconstituent: f - fraction of Then C0 = C f + (1- f ) C Pearlite f = (C0 - C Pearlite)/(C - C Pearlite) f Pearlite = 1- f You will figure out how to work it out for hypereutectoid compositions … What if cooled really fast? Faster cooling gives “non-equilibrium microconstituents” … Martensite, and more! Let’s measure the hardness of slow and fast cooled samples and compare… NEXT LAB : 11/03/06 NO GROUP REPORTS FOR THIS LAB!!!!