Paired with Lecture
advertisement

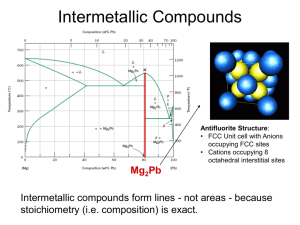
Isothermal Transformation Diagrams (Time-Temperature-Transformation (TTT) Diagrams) • Plot temperature on the y-axis • Plot time on the x-axis (typically logarithmic scale) • Maps of phase creation as a function time at temperature • These are ONLY valid for isothermal (constant temperature) transformation • Each diagram is ONLY valid for a specific composition Consider Eutectoid Transformation … g a + Fe3C Eutectoid transformation (Fe-C): 0.76 wt% C 6.7 wt% C 0.022 wt% C 1600 d L 1400 1200 g +Fe3C Eutectoid: Equil. Cooling: Ttransf. = 727ºC 800 DT 727°C a +Fe3C Undercooling by DTtransf. < 727C 600 400 0 (Fe) 0.76 ferrite L+Fe3C 1148°C 1000 0.022 a g +L g (austenite) Fe3C (cementite) T(°C) 1 2 3 4 5 6 6.7 Co , wt%C Isothermal Transformation Diagrams y, % transformed • Fe-C system, Co = 0.76 wt% C • Transformation at T = 675°C. 100 T = 675°C 50 0 10 2 1 T(°C) Austenite (stable) 10 4 time (s) TE (727C) 700 Austenite (unstable) 600 Pearlite isothermal transformation at 675°C 500 400 1 10 10 2 10 3 10 4 10 5 time (s) Effect of Cooling History in Fe-C System • Eutectoid composition, Co = 0.76 wt% C • Begin at T > 727°C • Rapidly cool to 625°C and hold isothermally. T(°C) Austenite (stable) 700 Austenite (unstable) 600 g g 500 TE (727C) Pearlite g g g g 400 1 10 10 2 10 3 time (s) 10 4 10 5 Hypoeutectoid TTT Diagram T(°C) d g L g +L L+Fe3C 1148°C a 0 1 (Fe) Fe3C g +Fe3C 727°C a+Fe3C 2 3 4 5 6 6.7 Co , wt%C • Af represents highest temperature ferrite can form • As is the eutectoid temperature • MS is the martensite start temperature Isothermal transformation diagram for 0.35% C, 0.37% Mn Reed-Hill, Abbaschian, Physical Metallurgy Principles, 3rd Edition, PWS Publishing Company, 1994. Hypereutectoid TTT Diagram T(°C) d g L g +L L+Fe3C 1148°C a 0 1 (Fe) Fe3C g +Fe3C 727°C a+Fe3C 2 3 4 5 6 6.7 Co , wt%C • Af represents highest temperature ferrite can form • As is the eutectoid temperature • MS is the martensite start temperature Isothermal transformation diagram for 1.13%C, 0.30% Mn Reed-Hill, Abbaschian, Physical Metallurgy Principles, 3rd Edition, PWS Publishing Company, 1994. Metastable Phase Transformations • Where on this diagram is martensite shown? • How about bainite? • How about spheroidite? This is the EQUILIBRIUM Phase Diagram for Fe-C system Metastable phases are temporary phase which are intermediate between the initial and equilibrium states Spheroidite T(°C) d L g +L g a (ferrite) L+Fe3C 1148°C Fe3C (cementite) a 727°C a+Fe3C 0 1 (Fe) 2 3 4 5 Fe3C g +Fe3C 6 6.7 Co , wt%C 60 m • Equilibrium phase diagram tells us that the stable phase distribution in the two phase field is: a + Fe3C – typically as lamellar microstructural constituent pearlite • Is pearlite the lowest energy state – NO! With lamellar structure pearlite has a lot of interfacial energy • If we anneal a pearlite microstructure we will get a transformation to a new phase distribution that minimizes the energy of the system when atomic mobility is activated Spheroidite: -- a grains with spherical Fe3C Martensite Transformation • Formed by rapid quenching of an alloy • Occurs in several alloy systems (indium-thallium, titanium, nickel-iron, gold-cadmium, and Steel) • Shear driven atomic realignment – similar to deformation twinning only more complex • Militaristic transformation – diffusionless transformation • Only driven by changes in temperature -- DG • New lattice is formed around a habit plane (plane shared between parent and daughter phases) • Form lens-shaped shear plates during transformation – speed of transformation can approach speed of sound in the material • Congruent phase change Martensitic Transformation Bain distortion: conversion of one lattice into another by expansion or contraction along crystallographic axes Lattice parameters change as we increase the amount of carbon in solution Reed-Hill, Abbaschian, Physical Metallurgy Principles, 3rd Edition, PWS Publishing Company, 1994. Martensite Effects • Change in volume associated with formation of martensite • For a 1% carbon steel we see a volume increase of 4% • The shear transformation and the volume change combine to create a high density of dislocations • Lath martensite has internal dislocation density on the order of 1015 – 1016 /m2 • Very fine microstruture of cell boundaries and laths Mechanical Properties Source: H. K. D. H. Bhadeshia, Bainite in Steels, 2nd Edition, Cambridge Press, 2001. Bainite • Bainite: --a lathes (strips) with long rods of Fe3C --diffusion controlled. • Isothermal Transf. Diagram 800 Austenite (stable) T(°C) A pearlite/bainite boundary 100% bainite 400 B A 200 10-1 10 103 a (ferrite) TE P pearlite 100% 600 Fe3C (cementite) 105 time (s) 5 m Tempering Martensite • reduces brittleness of martensite, • reduces internal stress caused by quenching. TS(MPa) YS(MPa) 1800 1400 TS YS 1200 1000 60 50 %RA 40 30 %RA 800 200 400 9 m 1600 Adapted from Fig. 10.33, Callister 7e. (Fig. 10.33 copyright by United States Steel Corporation, 1971.) 600 Tempering T (°C) • produces extremely small Fe3C particles surrounded by a. • decreases TS, YS but increases %RA Alloying Additions Effect of adding other elements Change transition temp. Cr, Ni, Mo, Si, Mn retard g a + Fe3C transformation 4340 steel (alloyed steel) Cooling Curve plot temp vs. time