Lesson 5.4 gas stoichiometry
advertisement

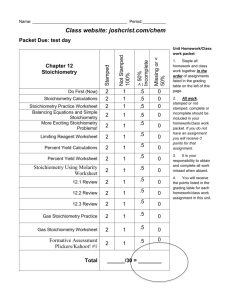
Lesson 5.4 Gas Stoichiometry Problems Suggested Reading Zumdahl Chapter 5 Sections 5.5 Essential Questions How is stoichiometry applied to gas problems? Learning Objectives Perform stoichiometric calculations involving gases. Apply the concept of molar volume at standard temperature and pressure in calculations. Introduction In Chapter 3 you learned how to find the mass of one substance in a chemical reaction from the mass of another substance in the reaction using stoichiometry. Now that you know how to use the ideal gas law, we can extend these types of problems to include gas volumes. You must use your dimensional analysis skill to be successful with gas stoichiometry! Gas Stoichiometry Gas stoichiometry is best learned by example, so consider the following reaction, which is often used to generate small quantities of oxygen gas: Suppose you heat 0.0100 mol of potassium chlorate in a test tube. How many liters of of oxygen can you produce at 298K and 1.02 atm (Side note: What type of reaction is given above? What is happening to the reactant? This is what you must start paying attention to in order to succeed on the chemical reactions section of your exams.) You solve such a problem by breaking it into two problems, one involving stoichiometry and the other involving the ideal gas law. You note that 2 mol KClO3 yields 3 mol of O2 (2:3 mole ratio). Therefore, Now that you have the moles of oxygen produced, you can use the ideal gas equation to calculate the volume of oxygen under the conditions given. Rearranging the ideal gas equations and substituting gives: Example: Using Molar Volume A sample of nitrogen gas has a volume of 1.75 L at STP. How many moles of N2 are present? (Please note: You can only use molar volume when the prompt specifies STP.) Solution: Applying the molar volume concept gives HOMEWORK: Please look at sample exercises 5.12, 5.13, and 5.14 on pages 191 - 193 for additional examples. Don't skip this step! Also, refer to lesson 5.3 for homework related to gas stoichiometry.