Electrochemistrynotesmp
advertisement

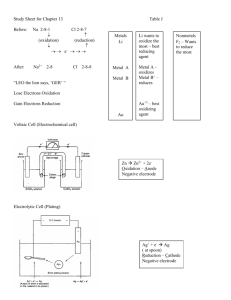
Electrochemistry o REDOX (reduction-oxidation) reactions involve the transfer of electrons. This transfer can release as heat or energy. o Moving electrons = current o There can be no reduction of a substance without an oxidation OIL: Oxidation is loss of electrons and thus an increase in oxidation state RIG: Reduction is the gain of electrons and thus a decrease in oxidation state An Oxidizing Agent is the reactant that is getting reduced, the substance that is causing the oxidation process to occur A Reducing Agents is the reactant that is getting oxidized, the substance that is causing the reduction process to occur Calculating Oxidation states o all atoms in their standard state at room temperature have an oxidation number of O o Oxygen will usually have an oxidation number of -2 unless working with the peroxide ion in which case the oxidation number is -1 o Fluorine always has the oxidation number of -1 o Hydrogen will have an oxidation number of +1 when it is with other nonmetals and a -1 when its combined with a metal o In a neutral molecule, all oxidation numbers will total to a net charge of zero o In a polyatomic ion, all oxidation numbers will total to a net charge of the polyatomic ion Practice: State the oxidation numbers for the underlined atom BeCl2 AgBr NO BaCrO4 NH4Cl To balance a REDOX o Not only must you balance the reaction to satisfy the law of conservation of mass BUT you must also make sure the charges are balanced as well o In simple REDOX reactions, the balancing of atoms with coefficients balances the charge as a result ____ Fe + ____ CuSO4 ____ Fe2(SO4)3 + ____ Cu 2 Fe + 3 Cu+2 2Fe+3 + 3Cu o In complex, REDOX reactions, the half reaction method is the best approach. Complex REDOX reactions o You must identify the oxidation states of each atom in the reactions o Identify the atom that undergoes oxidation and the one that undergoes reduction o Create a half reaction for each process. o Balance all atoms other than H and O o In an acidic reaction, add water to balance the oxygen atoms o Add hydrogen ions to balance the hydrogen o Balance the number of electrons in each reaction o Combine the reactions and simplify o Check to make sure all atoms and charges are equal on both sides o If basic solution, do all of the above steps except after you combine the reactions, Add hydroxide ions to both sides to neutralize the hydrogen ions Let’s use the same simple redox to practice the half reaction method ___ Fe + ____ CuSO4 ____ Cu + ____ Fe2(SO4)3 Oxidation Half Reaction Fe Fe+3 Fe Fe+3 + 3e 2Fe 2Fe+3 + 6e Reduction Half Reaction Cu+2 Cu Cu+2 + 2e Cu 3 Cu+2 + 6e 3Cu Add the reactions together and simplify! Examples Balance the following REDOX reaction in acidic solution MnO4-1 + SO2 Mn+2 + SO4-2 Balance the following REDOX reaction in basic solution Br2 Br -1 + BrO3-1 Voltaic Cells o Also known as galvanic cell or a battery o Change chemical energy into electrical energy if reactants are separated o Electrodes are called the cathode and anode o A cathode is where reduction occurs (RED CAT) o An anode is where oxidation occurs (AN OX) o Electrodes are placed in separate cells o Electrons flow from the anode to the cathode through a wire o Need a salt bridge or porous disk to connect the 2 cells (beakers). This allows for exchange of ions so build up of charges in each half cell does not happen Calculating Cell Potential or Ecell o The potential difference between the electrodes provides the driving force that pushes the electrons through the wire o Measured in volts(v) by a volt meter 1V = 1J/C of charge o Cell potential must be positive for a spontaneous reaction o Standard E cell is calculated at standard conditions (25 C, 1 atm of gas, or 1M solution) o The standard reduction potentials are measured indirectly relative to a standard hydrogen electrode. They are given to you on the reference sheet. Notice they are REDUCTION values. o The more positive the reduction potential, the more apt to being reduced(better oxidizing agent. o Standard reduction potentials are intensive properties. They do not depend on the amount present. Coefficients are not important ! To calculate Cell Potential or Ecell o Write reduction half reactions for each element o Look at the chart to determine the voltage for each. o Identify the most positive value. This is the element that is most easily reduced and it will be considered the cathode o Reverse the other atoms reaction and reverse the sign. This is the element that will be oxidized and will be considered to anode. o Add the 2 values together. E cell = E red + E ox Line Notation o Anode components listed on left o Cathode components listed on right o Double line represents salt bridge or porous disk Cu(s)| Cu+2 (aq) ||Zn+2 (aq) | Zn(s) Examples 1. Which is the better oxidizing agent? Zn+2 or Pb+2 2. Which is the better reducing agent? Ag(s) or Au(s) 3. A voltaic cell has 2 half cells. The first half cell has a copper electrode in a 1.0 M copper(II) nitrate solution. The second half cell contains the tin electrode in a 1.0 M tin(II) nitrate solution. The salt bridge between the 2 half cells completes the circuit. Assume tin is at the anode. Draw this out and calculate the cell voltage. 4. In a voltaic cell, a zinc electrode is placed in a 1.0 M solution of zinc nitrate while a copper electrode is placed in a 1.0 M solution of copper(II) nitrate. A salt bridge is in place. Calculate the cell potential. Spontaneity of a REDOX reaction o You can decide if a REDOX reaction will occur spontaneously by finding the cell potential o If the cell potential (Ecell)a positive value, it indicates a spontaneous reaction o If the cell potential is a negative value, it indicated a non spontaneous reaction Relationship between EMF (Ecell )and Free Energy( G) o When delta G is negative, a reaction is considered spontaneous o When delta G is positive, a reaction is considered nonspontaneous o So when delta G is negative, E cell is positive G = - n F E n= moles of electrons F= faraday’s constant = 96500 C/mol or J/molV E = standard cell potential (v) Example5 : Calculate the standard free energy change for the following reaction Zn(s) + 2 Ag+1 (aq) Zn+2(aq) + 2 Ag(s) Nernst Equation E = E – (RT) lnQ nF At 25 C E = E – (.0592) log Q n n= number of electrons transferred during the reaction F= Faraday’s constant Q= reaction quotient Q = [P] / [R] E = cell potential at nonstandard conditions o Used to calculate the EMF (Ecell )under nonstandard conditions o All concentrations are 1M in standard potentials o Most important idea to this equation: As the concentration of the products of a REDOX reaction increase, the cell voltage decreases; As the concentration of the reactants in a REDOX reaction increase, the voltage increases o As cells discharge, concentration changes and so does Ecell o Cells spontaneously discharge until they achieve equilibrium, the cell is “dead” Example 6: calculate the cell potential if the concentration of the zinc solution is 1.0M and the concentration of the copper solution is .10M Zn(s) + Cu+2 (aq) Zn+2(aq) + Cu(s) Electrolysis o Electrolytic cells require a voltage source to allow a nonspontaneous reactions to occur o Forcing a current through a cell to produce a chemical change for which the cell potential is negative Voltaic Cell Source of electrons are from the oxidation at the anode to the cathode spontaneously Cathode is positive; Anode is negative Electrodes are placed in 2 cells Electrolytic Cell Source of electrons are from a dc power supply which forces the electrons from anode to cathode Cathode is negative; Anode is positive Electrodes are placed in 1 cell with molten or a salt solution o In both types, oxidation takes place at the anode and reduction takes place at the cathode o In order for electrolysis to start, a minimum voltage is required. Diagram of an Electrolytic Cell Electroplating o o o o Thin coat of metal plates out on the surface of another. The metal to be plated is at the cathode Current: amount of charge per time. It is measured in Amperes(amps) 1 Amp equals 1C/Js I = q/t I = current(amps) q = charge (coulombs) t= time (seconds) Stoichiometry of the electroplating process: How much metal can plate out? 1. Convert current and time to quantity of charge in coulombs. (amps x time) = coulombs 2. Convert amount of charge in coulombs to moles of electrons Coulombs x 1 mole e= mole e96,500 coulombs 3. Convert moles of electrons to moles of substance Mole e- x mol substance = mol substance mole e4. Convert moles of substance to grams of substance Moles of substance x molar mass = mass of substance Examples: 7. 10.0 amps of current is passed through an electrolytic cell filled with molten LiCl for 500. Seconds. What is the mass of lithium collected at the anode? 8. 2.65 g Ag are collected at the cathode in an electrolytic cell filled with aqueous silver nitrate. How long does it take to collect this much if .25 amps are applied? Electrolysis of Water o Requires the presence of a soluble salt or dilute acid to serve as an electrolyte Some Commercial Electrolytic Processes of Importance are a. b. c. d. production of aluminum from the bauxite ore electro refining of metals metal plating like chrome electrolysis of sodium chloride